Wikipedia talk:WikiProject Elements/Archive 49
| This is an archive of past discussions on Wikipedia:WikiProject Elements. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 45 | ← | Archive 47 | Archive 48 | Archive 49 | Archive 50 | Archive 51 | → | Archive 55 |
RfC on group 3
- 20 July 2020: There appears to have been initiated an RfC on group 3, and it has been cancelled early. -DePiep (talk) 20:39, 24 July 2020 (UTC)
- Yes, Double sharp cancelled it. Sandbh (talk) 23:58, 24 July 2020 (UTC)
- All fine. I'm just making a record here. -DePiep (talk) 00:07, 25 July 2020 (UTC)
- Sandbh please stop messing artound with other persons edits. ~Just a reply does it. -DePiep (talk) 01:52, 25 July 2020 (UTC)
- Yes, Double sharp cancelled it. Sandbh (talk) 23:58, 24 July 2020 (UTC)
@DePiep: I was responding to your request to:
- "…please stop spreading loose threads re group 3 like this. Why not add it to a reasoning/thread?"
Thus, no more loose threads; they're in one place now. Sandbh (talk) 02:26, 25 July 2020 (UTC)
- Fair enough, but I saw threads changing & missing while working here, which is a weird experience. -DePiep (talk) 09:31, 25 July 2020 (UTC)
Regarding following the literature: astatine
Just something I mentioned in the previous discussion that may be worth considering.
Polonium is called a metalloid in the literature more often than astatine. However we call astatine a metalloid on Wikipedia but not polonium.
It may be worth for this reason recategorising astatine as a post-transition metal for the following reasons. This would give a "metalloids" category of {B, Si, Ge, As, Sb, Te} that more closely matches the literature: it only takes the most commonly accepted elements. It would also match Corson et al.'s original 1940 conclusions as well as Hermann et al.'s 2013 prediction (10.1103/PhysRevLett.111.116404), so it also results in a treatment of astatine that matches the literature about it. Scientifically this is also quite plausible as astatine seems to behave like a typical heavy metal, based on the sources at Metalloid#Astatine. The nonmetallic properties given there are unconvincing as the widely quoted melting and boiling point figures in fact contradict the one experimental determination we have, the calculated band gap energy contradicts the later calculation that At is a conductor, and a predominantly anionic aqueous chemistry is clearly not something that rules out metallicity considering that tungsten has such as well.
Taking the view that anything other than metals is a nonmetal (and thus that metalloids are a type of nonmetal), this would likely also have the benefit of allowing a one-sentence definition of what a metal is, based on discussions with Droog Andrey.
A metal is an element that has a Fermi surface even when amorphised.
Without the proviso, it is basically the solid-state physics definition, which helps make this definition well-grounded. The proviso kicks out carbon and arsenic which are obviously unwanted inclusions chemically speaking.
It also kicks out antimony. I still adhere to the view that the chemistry of antimony is about on the borderline between metals and nonmetals. (Let's not argue about cations, they are clearly not necessary and quite probably not sufficient for an element to be a metal. Rhenium is a metal and its cations have less reality than antimony's; germanium is by any sensible definition a nonmetal but may well form one.) However, since this (for obvious reasons) does not really give you a conclusive categorisation, I now think the fact that it is physically speaking not a real metal should decide. Well, bismuth is not a real metal, in the most stable state at standard conditions tin is even more so not a metal either, so antimony may be given up. This being said, I think anyone writing stuff about the p block metals would do well to consider antimony for comparison to illuminate trends, just like anyone writing stuff about the p block nonmetals would do well to consider bismuth for comparison.
We do not have to worry about copernicium being an insulator far away from the line because we do not have solid experimental evidence of how it behaves at the moment. We may worry about it when we do.
Therefore I claim this minor correction (At from metalloids to post-transition metals) would be beneficial both in terms of the science involved and in terms of following the literature's claims about astatine and its general treatment of which elements are metalloids.
So I propose to change from this:
to this:
Nothing in the layout has changed, only the categorisation of one single element. Double sharp (talk) 08:15, 22 July 2020 (UTC)
- It’s been seven years since condensed astatine was predicted to be a metal. 32 citations later there have been zero objections. We made a call on polonium as a metal rather than a metalloid. I agree making a call on astatine as a (post-transition) metal would now be reasonable. Sandbh (talk) 12:21, 22 July 2020 (UTC)
- Since the two of us are in agreement for this one, I guess there will not be any objection if we change it. So I've started doing so. Double sharp (talk) 10:00, 23 July 2020 (UTC)
OK, should've gotten most of them. @DePiep: could I trouble you to implement this on File:Simple_Periodic_Table_Chart-en.svg? Thanks! ~(talk) 10:14, 23 July 2020 (UTC)
- Will have to read this trough, to change a 400-day-plus discussion we had this fast. (Also: littl;e time these days, and engaging in other urgent PT talks). -DePiep (talk) 22:45, 23 July 2020 (UTC)
 Done. -DePiep (talk) 15:18, 25 July 2020 (UTC)
Done. -DePiep (talk) 15:18, 25 July 2020 (UTC)
@Double sharp: Does amorphous grey tin have a Fermi surface? I presume not? Since a Fermi surface is something characterised at absolute zero, does this mean white tin is not a metal? From a chemistry-based perspective I like the fuzzy perspective along the line of the IUPAC definition of hydrogen bond. Rather than a black or white categorisation, the definition provides that the greater the number of criteria satisfied, the more reliable is the characterisation as a hydrogen bond (Arunan et al. 2011). Sandbh (talk) 05:24, 24 July 2020 (UTC)
- @Sandbh: Well since I wrote that I have been talking a bit with Droog Andrey again, so I think the definition needs some fixing. I just have not updated it here yet because I lack something better so far, but hopefully the tweaking will not make it too bad. Tin I think can be dealt with by appealing to the standard state of white tin even if it is not the most stable at 0 K, but I need to check re amorphous white tin. More of a problem is that beryllium seems to be physically not a totally real metal either and is also getting near the border chemically. So I will have to think about how this should consistently affect borderline elements, particularly paying attention to tin, antimony, bismuth, polonium. So we must consider basicity, electropositivity, ionisation energy, reducing power also, correcting for oxidation states. Additionally coordination number, cf antimony being in same boat as polonium for this one. Aqueous cationic tendency is just suggestive, it must be applied to different nonmetals to fit the metals' character (maybe better use 3rd period nonmetals than 2nd period to avoid questions re metallicity of for example rhenium or gold). It is also reliant on coordination number (many of these p metals hydrolyse more than you would expect, their coordination numbers are low and therefore hydration structure is significantly distorted). So it is a delicate problem. Remember the problem of whether iodine or sulfur is a stronger nonmetal. So for now my working classification once again has antimony as a metal (pKa of oxide hydroxide is feeble, big gap between it and real nonmetals like germanium or tellurium), but we will see. This is not so clear but I think we can get a good intrinsic definition with preferably one criterion because simplest sufficient complexity. Better not multiply criteria without multiplying explanatory power for me. ;) Double sharp (talk) 06:50, 24 July 2020 (UTC)
The purported Ge2+ (aq.) cation
@Double sharp: Here is what our metalloid article says:
Whether or not germanium forms a cation is unclear, aside from the reported existence of the Ge2+ ion in a few esoteric compounds.
Sources mentioning germanium cations include:
- Powell & Brewer (1938) who state that the cadmium iodide CdI2 structure of germanous iodide GeI2 establishes the existence of the Ge++ ion (the CdI2 structure being found, according to Ladd (1999) in "many metallic halides, hydroxides, and chalcides");
- Everest (1953) who comments that, "it seems probable that the Ge++ ion can also occur in other crystalline germanous salts such as the phosphite, which is similar to the salt-like stannous phosphite and germanous phosphate, which resembles not only the stannous phosphates, but the manganous phosphates also";
- Pan, Fu & Huang (1964) who presume the formation of the simple Ge++ ion when Ge(OH)2 is dissolved in a perchloric acid solution, on the basis that, "ClO4− has little tendency to enter complex formation with a cation";
- Monconduit et al. (1992) who prepared the layer compound or phase Nb3GexTe6 (x ≃ 0.9), and reported that this contained a GeII cation;
- Richens (1997) who records that, "Ge2+ (aq) or possibly Ge(OH)+(aq) is said to exist in dilute air-free aqueous suspensions of the yellow hydrous monoxide…however both are unstable with respect to the ready formation of GeO2.nH2O";
- Rupar et al. (2008) who synthesized a cryptand compound containing a Ge2+ cation; and
- Schwietzer and Pesterfield (2010) who write that, "the monoxide GeO dissolves in dilute acids to give Ge+2 and in dilute bases to produce GeO2−2, all three entities being unstable in water".
Sources dismissing germanium cations or further qualifying their presumed existence include:
- Jolly and Latimer (1951) who assert that, "the germanous ion cannot be studied directly because no germanium (II) species exists in any appreciable concentration in noncomplexing aqueous solutions";
- Lidin (1996) who says that, "[germanium] forms no aquacations";
- Ladd (1999) who notes that the CdI2 structure is "intermediate in type between ionic and molecular compounds"; and
- Wiberg (2001) who states that, "no germanium cations are known".
- @Sandbh: Perchloric acid media studies seem still not really conclusive according to when I asked Droog Andrey about this. Therefore, I prefer to believe the theoretical study that shows it won't hydrolyse immediately. Nonetheless I still do not think this is so important for metallicity classification. Double sharp (talk) 06:44, 27 July 2020 (UTC)
Steudel's chemistry of the non-metals
Steudel R 2020, Chemistry of the Non-Metals: Syntheses - Structures - Bonding - Applications, De Gruyter,
This newest international edition is an updated translation of the latest German edition of 2013. I think the last English edition appeared in 1977. Sandbh (talk) 05:52, 2 August 2020 (UTC)
Defining a metal (chemistry perspective)
This is the last reasonable definition we discussed:
A metal is an element that has a lustrous appearance when freshly prepared or fractured and (a) has a densely-packed crystalline structure;1 or (b) forms a simple cation in aqueous solution;2 or (c) has a basic oxide. All other elements are nonmetals. 1 Hexagonal-close packed, face-centred cubic, α-lanthanum, α-samarium, body-centred tetragonal, or body-centred cubic 2 Including aqua-cations such as [Bi(OH2)8]3+
It's modelled after the IUPAC defintion of a hydrogen bond. The two physical and two chemical properties involved are mentioned in the literature as being characteristic of metals.
I draw a distinction here from e.g. an astronomy-based definition of a metal: "Hydrogen and helium are nonmetals; elements having Z > 2 are metals. Sandbh (talk) 05:21, 24 July 2020 (UTC)
- I have some thoughts but they will wait till I am sure they satisfy the standards I believe are relevant. So I just note that antimony seems to satisfy your definition as calculations suggest it does form an aqueous cation. Double sharp (talk) 07:08, 24 July 2020 (UTC)
I don't count [Sb(H2O)4(OH)2]+ as a simple cation. Sandbh (talk) 07:15, 24 July 2020 (UTC)
- @Sandbh: The unhydrolysed version [Sb(H2O)]~8]3+ also exists according to calculations. See 10.1016/j.cplett.2011.05.060 and 10.1021/ic901737y. Of course, first and second hydration spheres are strongly distorted, but that's exactly also the case for indium and tin aqua cations. Double sharp (talk) 07:22, 24 July 2020 (UTC)
I'll amend the definition to say:
- "…forms a simple cation,2 of significant concentration,3 in aqueous solution;"
- 3 Greater than trace level, in ambient conditions, within a normal pH range (0–14)
--- Sandbh (talk) 07:45, 24 July 2020 (UTC)
- @Sandbh: OK, now it gives what I think you want. I will still work on finding one I like more though. ;) Double sharp (talk) 08:01, 24 July 2020 (UTC)
I look forward to it.
Here it is completed then:
A metal is an element that has a lustrous appearance when freshly prepared or fractured and (a) has a densely-packed crystalline structure;1 or (b) forms a simple cation,2 in aqueous solution;3 or (c) has a basic oxide. All other elements are nonmetals. 1 Hexagonal-close packed, face-centred cubic, α-lanthanum, α-samarium, body-centred tetragonal, or body-centred cubic 2 Including aqua-cations such as [Bi(OH2)8]3+ 3 Of a concentration greater than trace level, in ambient conditions, within a normal pH range (0–14)
@Sandbh: Turns out, it is not hard to resolve to my taste. So here is my view.
This is not a chemical question, but a physical one, because it is about properties of simple substances rather than elements. We can talk about metallicity of NaK or lack thereof for NaCl just as one can for Na or Ge, it is about the bonding. But for NaK or NaCl they are not elements, it doesn't just relate to one element's chemistry.
The reason why it shows correlation with chemical behaviour for an element is because they both stem from the electronic structure giving the band structure, but again you can see differences. Germanium has an aqua cation (10.1002/jcc.21315; meanwhile this is super dependent on water as the solvent, if you change it iodine can have a cation as well) but is a semiconductor, tungsten is a metal but has no aqua cation. Close packing is structural, it's something similar to explain packing of Mn or Cu or Na or Sb vs packing of CsCl or NaCl or CuCl. So I don't consider most of these criteria relevant, they just show correlation because similar things drives both physical metallicity and chemical properties and thus creates chemical metallicity stereotypes. So I'd teach metals by the physical definition, and then say that because of what drives those physical properties to happen, they often share some chemical properties, but they're just tendencies with many many exceptions because that's not a defining criterion.
Therefore, I revert to the old Droog Andrey criterion based on the solid state physicists. Just one criterion will do. All elements that are metallic or semimetallic as simple substances, in all stable enough forms at STP, can be called metals as they have the important thing which is binding by a delocalised electron cloud. Re borderline elements:
- Alpha-tin is a semimetal, so no problem there. (The grey tin formed from the phase transition is amorphous.) It has a Fermi surface. Of course beta-tin presents no problems whatsoever.
- Germanium is a semiconductor, so not a metal. No need to worry about beta-germanium, that needs 120 kbar. At high enough pressure everybody is a metal, but that's not the important thing. That takes care of all other metalloids too.
- Carbon doesn't count as a metal even though graphite is a semimetal. Diamonds are forever after all, that surely meets the criterion for "stable enough". ;)
- Antimony is a metal, as the yellow and black allotropes are unstable. Light turns the yellow allotrope back into the metallic one, even at 0°C the black one turns into metallic antimony, see Holleman & Wiberg p. 758. That has to do with the large size of Sb atom.
- Arsenic is not, because black arsenic doesn't conduct electricity, yet needs 270°C for the conversion to gray arsenic to start, see Holleman & Wiberg p. 743.
As oganesson is bigger than tin I am in agreement with DA: intuitively, it seems more likely to be a (semi)metal. Copernicium just seems weird. So we wait for more predictions on period VII.
As such I feel this is only just one property that contributes to periodicity among many. Therefore, I restore the colouring of blocks as primary on my userpage. This is just for there for now of course.
You may disagree, of course. Double sharp (talk) 10:14, 26 July 2020 (UTC)
- The reference you gave for Ge2+ (aq) is a theoretical study. AFAIK there remains no empirical evidence for such a cation. Sandbh (talk) 13:03, 26 July 2020 (UTC)
- @Sandbh: It's good enough for me. Wulfsberg's generalisations imply this should have similar acidity to an average +3 d block ion, so it will not be impossible just from that. I suspect it is simply not known for sure because of (1) Ge being unstable in +2 state (it only stays there a while due to kinetic effects) and (2) maybe too ready complexation by other anions. As Droog Andrey suggested before, it could be tested for Ge2+ and Sb3+ and other doubtful transition metal cations with weakly complexing perchlorate or fluorocarboranate anions. Maybe it is interesting to find out for other reasons. But I think not for this classification, it is biased towards water. With different solvating ligands you may get iodine or phosphorus as cations.
- Again, you may disagree if you want. Double sharp (talk) 13:25, 26 July 2020 (UTC)
- From a chemistry perspective, the definition of a metal being something having a Fermi surface etc is too simple an answer to a complicated problem. As well, from chemistry-based perspective, I suggest a definition that includes some chemistry-based behaviour would be more appropriate.
- Your application of the Fermi surface definition is problematic.
- The most stable form of carbon is graphite, a semimetal.
- The most stable form of arsenic is the semi-metal form.
- The question of the status of amorphous grey tin remains outstanding.
- Packing efficiency is, I suggest, related to the Goldhammer-Herzfeld metallisation catastrophe. More specifically, the Goldhammer-Herzfeld criterion is the ratio of the force holding an individual atom's valence electrons in place with the forces on the same electrons from interactions between the atoms in the solid or liquid element. When the interatomic forces are greater than, or equal to, the atomic force, valence electron itinerancy is indicated and metallic behaviour is predicted.[1] Otherwise nonmetallic behaviour is anticipated. It is a simple measure of how metallic an element is, the recognised metalloids having ratios from around 0.85 to 1.1 and averaging 1.0.[2] As the ratio is based on classical arguments[3] it does not accommodate the finding that polonium, which has a value of ~0.95, adopts a metallic (rather than covalent) crystalline structure, on relativistic grounds.[4] Even so it offers a first order rationalization for the occurrence of metallic character amongst the elements.[5] Sandbh (talk) 13:37, 26 July 2020 (UTC)
- @Sandbh: You may have your opinion. I'm satisfied with how I handled carbon and arsenic in my original posting (anything metastable enough to persist at room temperature without obvious spontaneous transformation is good enough), and because it seems from the previous discussion that we start from too different premises on most things, I think a debate on those two will not be fruitful and will end up just generating more heat than light. As for tin, I do not know what "amorphous grey tin" might mean anyway because "amorphous" means "lacking crystalline structure", so I do not know what the difference between "amorphous grey tin" and "amorphous white tin" would be. Maybe I am wrong about that, in which case I would like a reference or two. Double sharp (talk) 13:41, 26 July 2020 (UTC)
- @Double sharp: There's nothing you need to change. The Fermi-surface definition, based on the behaviour of the elements at absolute zero, is a physics-based definition. What physicists refer to as metals is not the same as what chemists refer to as metals, as our metal article makes clear. Sandbh (talk) 14:06, 26 July 2020 (UTC)
- @Sandbh: Well, there you have it; I prefer the physics-based definition as metallicity or not is a property that can be applied to compounds, not just simple substances. You will find it in NaK just as surely as in Na or K. Whereas chemistry-based definitions are about properties of the elements, that's something different. Similar things give rise to both, that's why there are strong parallels, but as you can see they are not perfectly correlated (e.g. rhenium passes your physics criteria, fails your chemistry criteria). When an element gives rise to multiple simple substances (allotropes), I simply say that all allotropes metastable at room temperature must at least have Fermi surfaces: that's both a reasonable extrapolation of the physics-based idea and gives a metal-nonmetal boundary that chemists would also probably find not bad (metals Li-Be, Na-Al, K-Ga, Rb-Sb, Cs-At, Fr-Og maybe minus Cn and Og awaiting predictions). They may remove antimony if they wish as its chemistry has about equal relations to stereotype behaviour of metals and stereotype behaviour of nonmetals, and not being physically a real metal, I have considered it, but then as DA helpfully reminded me beryllium becomes a problem as it's not a true metal physically either (10.1103/PhysRevB.100.045145) and is also pretty weak chemically. So I decide to keep antimony there. Double sharp (talk) 14:14, 26 July 2020 (UTC)
- @Double sharp: There's nothing you need to change. The Fermi-surface definition, based on the behaviour of the elements at absolute zero, is a physics-based definition. What physicists refer to as metals is not the same as what chemists refer to as metals, as our metal article makes clear. Sandbh (talk) 14:06, 26 July 2020 (UTC)
- @Double sharp: Jones notes that in classification science, categories are usually defined by more than two attributes.
- In this context, I had in mind the following working definition for you:
- "An element is categorised as a metal if, in all of its crystallised bulk forms at or near ambient conditions, it has Fermi surface. Carbon and arsenic are precluded from this definition since they have stable semi-conducting allotropes (carbon as, for example, C60, and arsenic as arsenolamprite)."
- In this context, I had in mind the following working definition for you:
- I read the article on Be and saw nothing specifically there saying it's not a "true" metal, whatever that means. They observe Be is a nodal-line topological semimetal. 10.1103/PhysRevLett.117.096401 say Mg, Ca, and Sr also harbor Dirac Nodal Lines and speculate that the topological property of the DNL might naturally exist in other elemental metals as well. This appears to be scope creep to the point of verging on something less than useful, categorically speaking.
- Harrington (1946) referred to the true metals as being in groups 1 to 11.
- Klemm (1950) divided the elements into true metals, meta-metals, half-metals, and nonmetals. True metals were the alkali metals, the alkaline earth metals (excl. Be), the group 11 metals, and Al. Klemm counted Be as a meta-metal along with the group 12 and remaining group 13 metals, and beta-Sn, and Pb.
- Russell and Lee (2005, p. 158) more simply observe, as follows:
- "The cause of Be's low ductility is its proximity to the nonmetallic elements in the upper right corner of the periodic table, which results in mixed metallic and covalent bonding. In HCP Be bonding within the (0001) basal plane is metallic, but bonding between (0001) planes is partly metallic and partly covalent. This causes large differences in the critical resolved shear stresses among Be's three active slip systems and gives Be the lowest c/a ratio (1.56%) of any HCP element. The Poisson ratio of Be is only 0.02, much lower than the 0.25 to 0.40 Poisson ratios of most metals."
--- Sandbh (talk) 00:42, 27 July 2020 (UTC)
- @Sandbh: Well, that does eliminate some difficulties regarding checking amorphised forms of everybody (which is maybe hard to find in some cases), so yes, this does seem like a useful simplification. It excludes red phosphorus, but that will be a nonmetal no matter what anyway.
- "True" metal means as opposed to being a semimetal. I agree that this is not a useful distinction for these purposes because the important thing is the delocalisation and they have both. Beryllium is just an illustration of why it doesn't seem useful.
Antimony
- It is also why I continue to regard antimony as a metal. It is just too much trouble to exclude it. If one worries that it is a semimetal – then beryllium is in trouble (and not for very useful reasons). If one worries that its structure is not close packed and has only six nearest neighbours – then polonium is in trouble (and again not for what seem like very useful reasons). If one worries that it forms no aqueous cation or basic oxide – then rhenium is in trouble. If one worries that it has the unstable forms that are not metallic – well they spontaneously convert to the metallic form at standard conditions already, so they're hardly representative, and considering unrepresentative forms opens Pandora's box for me. So I prefer to leave it in because all the simple ways to exclude it give unwanted consequences, anything else is likely too complex, and I don't feel the gains are justified. Will the chemists complain in droves if antimony is regarded as a metal on physical grounds? I don't think so. I guess most chemists who don't use a "metalloid" category already do that. And besides antimony is noticeably more metallic than the other bunch of metalloids even if it is less so than bismuth. So simplest sufficient complexity in the form I prefer to apply it favours leaving Sb in AFAICS. That is if we start from my premise that this is a question more usefully answered from the physical perspective and simple substances.
- You may disagree based on the history, but you know I prefer to ignore the history when coming up with the classifications and just ask how we would do it if starting afresh now. Usually the result I get from that is "something quite like the history but maybe with a few points of minor difference", which seems reasonable to me: in the past they saw the same thing, so only minor differences (small enough that inertia sets in and people don't feel a need to change it as the theory doesn't go quite deep enough) will be observed. You saw it in my approach to the periodic table with He and Lu/Lr, you see it again now in metallicity with Sb. ;)
- P.S. From Metallium you can see how many elements look as rods. Unfortunately not all the key region is seen, but you can see how silicon and germanium do not have the metallic lustre of the preceding element, but a glassy mirror-like one. Antimony and bismuth have the metallic lustre. Tellurium has no rod listed, but it does look more glassy like selenium. Arsenic is not very clear in the sample though. For the other useful link: their element series coins (and descriptions). Apparently, bismuth and antimony despite being brittle do not shatter upon striking coins. Arsenic is not mentioned but also not offered for sale yet, so I presume the obvious approach fails to work. Tellurium was offered, but from hot-pressing instead. Double sharp (talk) 04:22, 27 July 2020 (UTC)
@Double sharp:Yes, it doesn't matter about antimony. It's fine to regard it as a metal, just as it's fine to put Lu in group 3, as long as the context is explained to readers, colleagues, and students. Then one can sit back and appreciate the perspective that this context offers and, if appropriate, compare it with other contexts, e.g. the one that treats the metalloids as the semimetals + semiconductors = B, C, Si, P, Ge, As, Se, Sb, Te, I, Bi.
It'd be useful to have good responses up one's sleeve to the following observations:
- "When non-metallic elements react with the oxidizing acids, acidic oxides or acids are formed… The trisulphides of arsenic and antimony are acidic, forming salts with yellow ammonium sulphide and alkali, while that of bismuth is typical of a metal." (Moody 1969, pp. 267, 321)
- "Interest centres on the trend from non-metallic to metallic properties with increasing atomic weight. Thus there are many parallels between phosphorus and arsenic, but considerably fewer between phosphorus and bismuth, which is a typical B metal like tin or lead. Arsenic and antimony are important largely because of their intermediate or metalloid character…" (Smith 1973, p. 547)
- "All the elements react readily with halogens but are unaffected by nonoxidising acids. Nitric acid gives, respectively, phosphoric acid, arsenic acid, antimony trioxide, and bismuth nitrate, which well illustrates the increasing metallic character as the group is descended." (Cotton & Wilkinson 1976, p. 288)
- "In the nitrogen family, we move from nonmetals that form acidic oxides—nitrogen and phosphorus—to metalloids that form amphoteric oxides—arsenic and antimony—to the last element—bismuth—that is barely a metal and forms a basic oxide." (Brady & Holum 1996, p. 61)
- "Antimony is found in forms analogous to those of arsenic… The most common antimony chalcogenide ore is stibinite (antimonite), Sb2S3… Some metal antimonides are breithauptite = NiSb… and dyscrasite (antimony silver) = Ag3Sb… Bismuth is not found in large amounts in nature… Unlike arsenic and antimony, it is not found as anionic bismuthides." (Wiberg 2001, p. 757)
- "Bismuth(III) oxide occurs naturally as bismite and is formed when Bi combines with O2 on heating. In contrast to earlier members of group 15, molecular species are not observed for Bi2O3 and the structure is more like that of a typical metal oxide.’ (Housecroft & Sharpe 2008, p. 474)
--- Sandbh (talk) 04:59, 27 July 2020 (UTC)
For you consideration, Yb is a topological semimetal, as well as the alkaline earths.
There is nothing to worry about the fact that Sb is a semimetal, so too is Bi.
The tricky part is that there are too many "ands" for apparent comfort, unlike Po which forms a cation in aqueous solution, unlike rhenium, which is close packed. Thus, in support of Sb as a metalloid:
|
|
|
In support of Sb as a metal:
- relatively high reflectivity: Si 41%; Ge 35%; As 46%; Sb 70%; Te 59%.
- fair electrical conductor;
- the liquid form is a metallic conductor;
- it can form alloys with one or more metals such as aluminium, iron, nickel, copper, zinc, tin, lead, and bismuth;
- displaces hydrogen from water, when heated
- the otherwise acidic pentoxide Sb2O5 shows some basic (metallic) behaviour in that it can be dissolved in very acidic solutions, with the formation of the oxycation "SbO+
2" - the compound Sb8(GaCl4)2, which contains the homopolycation, Sb82+, was prepared in 2004.
No need for a debate, nor do I have an expectation of one. Sandbh (talk) 07:25, 27 July 2020 (UTC)
Lu + metallicity
@Sandbh: Two parts to this post. First one is about group 3 (since you mention Lu in group 3 as contextual), and second one is about metallicity. I say things calmly (I think, if not you can scold me and tell me what to change) about group 3 because the approaches I take for both subjects seem parallel. You may skip that part or ask me to delete it if you feel it is off topic, I just wanted to draw the parallels. So let's start. It's not a debate, just a description of how I see both things.
Lu: Contextual or universal
I view Lu in group 3 as not contextual, rather I consider it universally applicable. However I also consider universally applicable the secondaries not explicitly shown by it, like Sc-Y-La and B-Al-Sc. Sc-Y-La is a valid linkage, it is totally valid secondary periodicity and useful in any context. And, so is B-Al-Sc, so is C-Si-Ti, so is He-Ne, so is H-F, so is even Ti-Zr-Ce. The working chemist should be aware of all of those and then some, no matter which field he is in. It's not just that some are more useful in this field, some in that field; they are actually all useful in all fields of chemistry. The continued popularity of the 8 column form in Russia (with B, Al, Sc all in one column) speaks to that.
Why I favour He-Be + Sc-Y-Lu is for reasons of pedagogy and consistency in requirements. It is quite easy to teach (let us say, not harder than standard He-Ne + Sc-Y-La), and avoids awkward questions from the back of the classroom about why Sc-Y-La is considered important enough to reflect as primary periodicity but not, say, B-Al-Sc, or why He-Ne is important enough but not H-F (just think of physical properties for that one). And the reason why I have to avoid such questions is because of my rejection of gas-phase ground-state configurations and condensed-phase configurations (that's why I don't appeal to electron configurations to remove B-Al-Sc from consideration, because after all there are legitimate similarities and it's something useful for chemists to know).
Because to my taste, those configurations are drilling down too deeply to something that will not be useful until the students reach a rather advanced level of chemistry. One can ask students to memorise the gas-phase exceptions (I did memorise them), but then they cannot use them to predict much outside some very specialised topics that occur very late and that most will not go on to, so then it feels like I'd be asking them for something that they'll probably never use. And it is hard to ask for memorising condensed-phase configurations, when solid state physics is generally not part of the high-school chemistry syllabus, when there are just fractions of many configurations added together, and the energy differences are so low that configuration may be impacted by turning on the lights (visible-light photons are enough). These two will be important for too few things for the first-year students getting a periodic table, I find.
And I want to be able to start the g block without getting a migraine painstakingly characterising a bear that looks like it wants lunch by some hard-to-observe property to borrow your analogy. And I think that's important because the kids need to get some wonder about how things are still being discovered, so when these elements appear I still think they should go (and they probably will go) on everyone's periodic tables. But you see, calculations already cannot yet determine lowest-energy configuration for single atom of element 123, and anyway in many such early g elements we have a bunch of electron configurations that are extremely extremely close in energy (sometimes there is really even no definite ground state, just fractions of one configuration plus fractions of another).
I just don't want to deal with all this complexity. It's good enough for almost all purposes to just look at how many valence electrons there are and which orbitals are valence orbitals to determine blocks, without actually going further and asking for an exact configuration. I guess it can be called simplest sufficient complexity. And the result isn't even bad. Lutetium is not further from scandium and yttrium than lanthanum, and helium over beryllium gives hydrogen over lithium a partner (and some understanding of kainosymmetry too for later; this way, no one has to unlearn stuff if they do choose to go deeper), so the table is sound enough for vigorous use both by beginners and by expert chemists.
Of course, one can add criteria to make current standard He-Ne + Sc-Y-La come out as the result, but again: do I think adding some more complexity in teaching the criteria for element placement is going to be worth the added benefits this might give? I think, to my taste, not really. And remember that I do ask to keep in mind that secondary periodicity exists and comes from having the same number of electrons above or below a noble gas configuration. So we don't lose sight of Sc-Y-La (all three electrons over a noble gas core).
We also don't lose sight of B-Al-Sc (same), C-Si-Ti (all four electrons above), P-V, S-Cr, Cl-Mn (same idea, five, six, or seven electrons), d elements to actinides (Y-Ac, Zr-Th, Nb-Pa, Mo-U), and of course He-Ne (all noble gas cores), H-F (both one electron short), and Be-Mg-Zn (all six electrons away from a noble gas core) and Ca-Sr-Yb (all sixteen electrons away from a noble gas core). Even stuff like Pt to chalcogens or Au to halogens are not neglected because I would mention that Hg has relativistically induced properties of a noble gas (so, same thing probably happens for Rg with halogenic properties and Nh as very two-faced like astatine).
So all important linkages are covered, they will help all chemists. And definitely, when discussing group 3, don't just cover Sc-Y-Lu-Lr, also please include not just La-Ac but also Al (maybe even boron to some degree) for comparison. That's why I feel it should be good for real chemists: I favour showing Sc-Y-Lu, but also teaching how to get this sort of thing to generally get not just Sc-Y-La but all those other things that are helpful like B-Al-Sc and C-Si-Ti. Those are all fine, and I ask to keep them in mind.
But I just treat the one from valence orbitals as primary for actually drawing. Because it is good enough, and based on very few criteria (two seem to do: one about valence electrons, and one saying that if subshells happen to get drowned within a block, then just treat them consistently as valent or non-valent when counting valence electrons). It is also very easy to motivate, teach, predict from, and use to classify future elements that we hope should come this decade, without having to do too much work. I don't want to teach multiple forms when I can take one that is very good in itself and just supplement it with secondary periodicity readings to get almost the same thing. And I don't want to have to restrict any generalisations because I want to take the simplest possible thing (so, start with very few things to remember) that will let you guess properties of almost everything quite well to build intuition. So, for me I just want to teach things that work everywhere. Local stuff that works only in some places is fine, but only later when they drill down to the part of the table they want to carve out for themselves.
- @Double sharp:What you have set out above is a considered approach to your preference for HeBe and Lu in group 3. Is there a condensed description for this interest dependence context e.g. "the periodic table in the context of valence orbitals" or something like that? Sandbh (talk) 00:10, 28 July 2020 (UTC)
- @Sandbh: You can call it that as a premise, that is a good name for it. I simply think it's so particularly useful as a premise (because of valence orbitals being key to chemistry) that it is worth making universal, as you know. We will not lose the secondary relationships by doing so, as we know how to read them off (same distance from either side of the table but different columns). Just some examples below courtesy of DA.
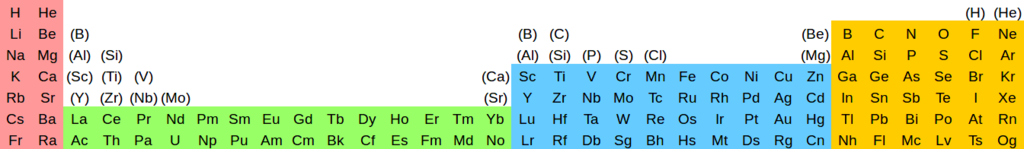
- Of course, you can already read those off even without the parentheses as I said above. I just think this is a far more universal premise than any other. In chemistry valence orbitals are ubiquitous in their importance. Ground state electron configuration is only important for some very specialised topics involving gas phase atoms (we have discussed that, that is what I understand from reliable sources, if you want you may disagree), and then you will want to show the anomalies like Cr and Cu as well, not only La and Ac. So, that's why I don't think there are very many contexts in which He-Ne + Sc-Y-La is important. My opinion: why just those two secondary periodicities? Maybe He-Ne is exceptional, but if Sc-Y-La is important, then probably B-Al-Sc or Be-Mg-Zn cries out for some attention too. That's how I think. Again you are free to disagree. Double sharp (talk) 03:28, 28 July 2020 (UTC)
Metallicity
So, how do I deal with metallicity? I guess the answer comes from how I would respond to your observations.
I would not really lose sleep over the fact that Sb has some significant chemical properties that are more characteristic of the nonmetals, because otherwise the situation becomes a bit awkward for the more electronegative metals that favour higher oxidation states. If we demand basic oxides, or formation of oxoacid salts, then questions will arise about niobium or tantalum which have neither. If we complain about antimonides, then we face the problem that polonium is found in nature as polonides (Po decays to Pb, ergo lead polonide appears in nature). If we complain about reactions with nitric acid, then questions arise about tin, because while dilute nitric acid forms tin nitrate, concentrated nitric acid produces stannic acid. If we complain about molecular species for oxides, then questions arise about rhenium or osmium in high oxidation states when they are at their most electronegative.
In particular, Sn seems also dangerously close to the border. The stable state at STP of 0°C is a semimetal (can even be called a zero band-gap semiconductor) that has only four nearest neighbours (conversion of beta to alpha form will already happen), it has no basic oxide, and its simple cation is badly hydrolysed (you won't find a more hydrolysed +2 cation except maybe uncertain germanium).
Meanwhile it will become a bit difficult to classify even the already known superheavy elements even provisionally from predictions because there are a lot of things to think about and we don't even have all properties predicted yet (crystal structure predictions are still not around for Mc, Lv, and Ts, and I am not aware of any predictions about the aqua cations of elements past Rf).
So, again: I do not want to have to deal with this complexity, it's starting to become a similar problem to the above issues for electron configurations. I do not want to force the students to memorise a lot of different things for different parts of the table (especially because, well, how many chemists actually focus on the whole table in their work?), when a single generalisation could get almost everything across and draw a simple line that they can use. They can drill down later when they decide where in the table they will make their home. But for beginners I prefer stuff that generalises everywhere, as it will be the most useful to start from.
So I apply the same principle as I did to group 3 to resolve metallicity for my taste with basically two criteria (one about Fermi surfaces, one about stable allotropes). And that will immediately let me say provisionally that the whole 7th period may just as well be called metals (except maybe copernicium, 6d expansion appears not to be enough) due to big atomic radii, and probably the whole 8th period is metallised for the same reason except element 172 because of closed-shell (probably a dispersion-force solid or liquid like a tri-xenon).
I do not want to complicate the definition with details to kick out antimony, when the result of the very simple definition looks good enough. Antimony is very weak as a metal, yes, but maybe the logical endpoint after tin and bismuth's steps towards nonmetallicity (all other metalloids are more nonmetallic). And it does not look totally out of place. DA mentioned that in Russian textbooks Sb is often treated as a metal (there are no metalloids), and I doubt anyone complains much.
As a parallel to secondary periodicity I of course would teach that near the borderline elements will have some echoes of the other side, and then happily go along adding elements on the wrong side when describing "the nonmetals" (where I would always try to include at least tin, astatine, polonium, bismuth, maybe even lead for comparison) as well as "the metals" (where I would probably include for comparison all typically recognised metalloids plus selenium). Silicon and germanium conduct electricity like metals when melted, antimony stays metallic, but arsenic loses metallic conductivity. Tin, antimony, and bismuth all have significant nonmetal-like chemical properties, so do many transition metals in high oxidation states. Some metal-like chemistry is observed with germanium in the lower oxidation state +2. All of that is useful to know too.
For comparisons, therefore, elements coming out a little to the wrong side can be included, but the usual line I draw remains useful enough for most work and not complex to motivate. So I take simplest sufficient complexity and say "well, Sb and only Sb added from metalloids to metals is not so bad I want to lose sleep over it". There's no need to have lots and lots of different metal-nonmetal lines when we can just draw one and say "of course, keep in mind that elements near the line can have both characters". That's again my parallel to secondary periodicity; yes, a good chemist must keep relationship of B-Al-Sc-Y-La in mind, just as a good chemist should not let a metal-nonmetal difference blind him to similarities like As-Sb-Bi, the nonmetallic properties of Sb, or the metallic properties of Ge. But again: a short and simple definition and line is always there for you to start from and treat as primary for teaching and showing.
So, for me: is Sb as a metal universally applicable? Yes, I would say it is, because it's useful to keep in mind, and is sound enough as a classification for vigorous use by both beginners and expert chemists. So, of course, is Sb as a nonmetal: all chemists seriously working with it must be aware of both sides of the coin. And a book on elements split into metals and nonmetals would do very well covering Sb in both parts. I simply put the former as primary because it makes the definition less complicated. You can see that what I do here is basically parallel to how I handle group 3.
Again, that's all my view, I simply describe it, hopefully calmly. You may disagree if you want. Double sharp (talk) 10:18, 27 July 2020 (UTC)
- @Double sharp: This is all good except I don't understand what the simple line is. Is it the useful simplification I suggested?
- Tin, being ductile and forming a simple cation (aq.), is a distraction, so to speak. I suggest another distraction is STP (0 °C 100 kPa). Not much aqueous chemistry will occur in ice. More meaningful is SATP (25 °C 100 kPa). Compare with the NTP of NIST: 20 °C, absolute pressure of 1 atm (14.696 psi, 101.325 kPa). Sandbh (talk) 00:31, 28 July 2020 (UTC)
The simple line
- @Sandbh: Yes, it is your suggested improvement to my definition (still including antimony). So my metal-nonmetal line cuts off H from Li, He from Be in the s block; and then within each period of the p block it goes between Be and B; Al and Si; Ga and Ge; Sb and Te; At and Rn. I don't yet really know where it'll be in the seventh period, so I wait for more predictions. (I cannot believe oganesson as a semiconductor, intuition revolts and says that if it's larger than tin in terms of atomic radius it should get metallised; as for copernicium, well, all depends on how active 6d orbitals turn out to be.)
- Tin pest just worries me because the transition can still start above freezing point of water. Regarding Sn2+: be careful of this cation. ;) Coordination number is small, the metal cation is very acidic, because lone pair is polarising and water molecules are forced into positions where they easily lose protons (same problem as Sb3+), see 10.1021/ja039248p. Actually aquated Sn2+ is not even known in the gas phase, it seems to exist in condensed phase only due to thermal motion of water molecules. Same problem happens with Pb2+, so likely also with Bi3+. Double sharp (talk) 03:59, 28 July 2020 (UTC)
- @Double sharp: I'm OK with Sn2+. I can see it on the E-pH diagram, within pH 0–1, straddling either side of 0.0 E(volts). Even as grey tin it still has a Fermi surface, since it's a zero-gap semimetal. Sandbh (talk) 07:13, 28 July 2020 (UTC)
- @Sandbh: And is Sn4+ also on those E-pH diagrams? Double sharp (talk) 07:16, 28 July 2020 (UTC)
- @Double sharp: Yes, it is. pH <= −0.5; 0.25–3.0 V.
- PS: Chemically, grey tin falls marginally on the metal side of the line given tin is ordinarily regarded as chemically weak metal rather than a chemically weak nonmetal. Sandbh (talk) 07:21, 28 July 2020 (UTC)
- @Sandbh: And does that seem convincing to you considering that it surely must be more acidic than Zr4+ by Fajans' rules? It doesn't to me, but maybe you disagree. Double sharp (talk) 07:25, 28 July 2020 (UTC)
- @Double sharp: You’ll have to elaborate what it is that is supposed to be convincing, or not. Sandbh (talk) 11:22, 28 July 2020 (UTC)
- @Sandbh: The presence of Sn4+ on the E-pH diagrams. Double sharp (talk) 11:26, 28 July 2020 (UTC)
- @Double sharp: I see the same patterns in the Atlas of Electrochemical Equilibria in Aqueous Solutions. The Pourbaix authors refer to, "The ion Zr++++, which has been identified only in concentrated solutions of very low pH". The tin authors don't say anything notable (that I could understand) about Sn++++.
- Parish says of the 4d- and 5d- metals (Zr to Cd; Hf to Hg) that most redox potential data refer to complexes rather than aquated species (p. 133). The most important species in acidic solution is the tetrameric cation, Zr4(OH)88+. There is no evidence for mononuclear species, and the 'zirconyl' ion, ZrO2+, seems definitely to have been disproved. (p. 135). Lidin (1996) says Zr forms no aqua-cations; Sn forms the Sn2+ cation in a highly acidic medium in the form of the complex [Sn(H2O)3]2+.
- He includes Sc and Y in the chapter on the 4f metals. He says the 4f metals have a very simple chemistry since with but few exception only one oxidation state is displayed, viz. +3. In this respect they resemble the s-block elements, and indeed there are pronounced similarities to Ca, which often occurs in Ln minerals, and to Sr and Ba, particularly in the complicated solid-state structures found. (p. 143).
- Parish goes on to say of the 4d- and 5d- metals that, "all the compounds show considerable covalency, and descriptions in terms of the ionic model are likely to be rather inaccurate." (p. 137) --- Sandbh (talk) 13:56, 28 July 2020 (UTC)
- @Sandbh: Basically, I cannot believe it myself because Sn4+ has about equal size to Zr4+, and Sn is more electronegative than Zr. For simple reason of Fajans' rules it has to be more polarising, so it does not make sense that there would exist Sn4+ but not Zr4+. Recent calculations and experiments support what I say here. In perchloric acid medium, if concentration of Zr is kept low (to avoid tetramerisation) and [H+] kept high (1-2 M), you will get [Zr(H2O)8]4+. In review of aqueous metal cations (10.1351/PAC-CON-09-10-22), both [Zr(H2O)8]4+ and [Hf(H2O)8]4+ were included (along with Th, U, Np, Pu +4 cations): and this even when Sb3+ is excluded from metals: of course it is mentioned that they are prone to hydrolysis and tetramerisation. From the paper doing calculations (also on Sb), it appears we indeed have experimental evidence for Zr and Hf cations as we do for Ce and U (just make pH low). That's a far cry from what they calculate for Ge4+, Sn4+, and Pb4+ (hydrolysis within picoseconds).
As for covalency: that is, as we have discussed already at great length, just oxidation states. You will find more ionic character if you take electronegative anions and force the metals into low oxidation states. Zirconium and hafnium have very simple main-group-like chemistry too, as with few exceptions only +4 is displayed. Same story for niobium and tantalum with +5. Greenwood and Earnshaw say it. "Lower oxidation states are rather sparsely represented for Zr and Hf" (p. 958), "most of the chemistries of niobium and tantalum are confined to the group oxidation state of +5" (p. 979). In 4d and 5d metals, real transition properties only start strongly at molybdenum and tungsten with cluster compounds in lower oxidation states. We have gone over this. If you want to disagree, that's fine. Double sharp (talk) 14:23, 28 July 2020 (UTC)
- @Double sharp: I have no concerns wrt to Zr, Hf, and Sn cations.
- On covalency and oxidation states, Parish writes wrt the 4d- and 5d- series (excl. group 3), "In their compounds a wide range of oxidation states is shown, but all are characterised by the formation of bonds of high covalent character, and there are no simple ionic compounds. Only in a few cases are even simple aquated cations known, other ligands, often anions, being bound in preference to water." (p. 112)
- The very simple main-group-like chemistry of Zr and Hf is more like the very covalent chemistry of the p-metals. Thus, Parish writes: "The most striking aspect of the chemistry of the p-block metals is the pronounced tendency to covalency." (p. 192)
- In her entry for Zr, Rossotti writes, "As we shall find with other members of the 2nd and 3rd transition series, simply hydrated cations are fairly scarce." (p. 337)
- On Hf, she writes, "between La and Hf come the fourteen 4f elements…this Ln contraction has repercussions beyond the 4f series and one of its effects is that the elements of the 3rd transition series…have (except for La) radii that are very similar to those with the same value of n in the second transition series…" (p. 461)
- Talbot and Talbot (2019, p. 336), in Corrosion Science and Technology, 3rd ed., write, "The only important oxidation states are Zr(IV) and Hf(IV). The charge density that would result from removing all four valence electrons is so high that compounds of Zr(IV) and Hf(IV) are mainly covalent."
- On Sc, Rossotti writes, "Its chemistry is largely, but not entirely that of the Sc3+ cation"; for Y, "…behaves very much like Sc"; for La, "…forms predominately ionic compounds"; for Ac, "…might also be expected to resemble La as the launch pad element for a series of successors with increasingly filled f orbitals."
- I do like the phrasing Rossotti uses viz. "repercussions beyond the 4f series", and "launch pad element".
- PS: Rossotti notes ScH2 is a metallic conductor and asks if it can be Sc3+.2H− with a free electron in the conduction band. That is how Wiberg formulates it. On CsScCl3 she asks if it does really contain ScII.
- --- Sandbh (talk) 02:07, 29 July 2020 (UTC)
- @Sandbh: We have gone over this, with sources, and going over it again will not be helpful. So I stop here rather than argue. Thank you for the suggestion for the definition, I have put in something like it on my page, but I continue to regard Zr-Hf and Nb-Ta as s-like pre-transition, just modified for higher oxidation states. Not so much difference between them and Th and Pa, really. Double sharp (talk) 02:36, 29 July 2020 (UTC)
- @Double sharp: That's fine. It's all water under the bridge, or food for thought (or not). Sandbh (talk) 06:23, 29 July 2020 (UTC)
Antimony
- P.S. Chemically speaking, when a "metalloids" category is not used, Sb usually goes to the metals (that's standard with Russian textbooks). Double sharp (talk) 07:37, 28 July 2020 (UTC)
- That’s fine as long as they explain the context for so doing, given Sb has a predominately non-metallic chemistry. And how do they treat As?
- For what it is worth, I have a Russian textbook, General Chemistry, 3rd ed., by N.L. Glinka, Mir Publishers, Moscow, 1980, English translation. Glinka says N-Bi are characterised as a whole as non-metals. Antimony has nonmetallic and metallic properties to an equal extent, and in Bi the metallic properties predominate over the non-metallic ones. (Vol. 2, pp. 66–67). [He treats Group 3 as Sc-Y-La-Ac.] Sandbh (talk) 11:22, 28 July 2020 (UTC)
- @Sandbh: I don't know, I got the info about Sb from DA. Probably As is a nonmetal following staircase (which he said is usually drawn) and as he said serious sources there call Ge one as well. Glinka says what DA said about Sb, I see. Double sharp (talk) 11:26, 28 July 2020 (UTC)
Citations
References
Back in 2018
I proposed a simple criterion: Neighbours
| “ | Look at chemical bonds formed by element E with itself and its two closest neighbours in the sense of atomic number. If metallic phases prevail, E is a metal. If covalent or ionic phases prevail, E is a reactive non-metal. If bond absence prevails, E is a noble gas. |
” |
Droog Andrey (talk) 11:48, 3 August 2020 (UTC)
About the hassium TFA blurb
FA Hassium is to be TFA on Oct 9. This is the blurb.
I see a problem with the current image in there. The image is a periodic table cell of Hs, newly drawn. However, it does not resemble any cell format in use at enwiki. Even the name is omitted. I don't see why we would redesign the cell for a blurb while we have stable standard forms in use. So I propose to use an existing cell form, resembling our regular Periodic table cell. I note this here because this would be a deviation from our stable standards for no clear reason. Talk is here. -DePiep (talk) 19:55, 5 October 2020 (UTC)
The periodic system landscape
| Physics- philosophy |
|||
|
|||
| Chemistry | |||
Scerri (2020) talks about a continuum of periodic systems, with Rayner-Canham's unruly inorganic chemist's table at one end, and the left-step or Platonic periodic table at the other end. Somewhere in the middle is the currently popular 18-column table.
This is the physics—chemistry axis.
Cao et al. (2019) talk about chemical, pedagogic, and designer periodic tables.
This is the didactic—designer axis.
Combining the two axes results in a 3 × 3 grid, as shown.
The example placements are rough first go’s.
I probably need to expand the grid into a 5 x 5, and place the systems on the basis of a score out of 5 for each axis. Sandbh (talk) 06:29, 20 July 2020 (UTC)
- Cao C, Hu H, Li J, and Schwarz WHE 2019, "Physical origin of chemical periodicities in the system of elements", Pure & Applied Chemistry vol. 91, pp. 1969–1999
- Scerri E 2020, The periodic table: Its story and significance, 2nd ed., Oxford University Press, New York, pp. 402–403
- There's much to explore here, but from start there is this re the Proposed standard classroom version:
The confusion results from the false premise that the 18-column periodic table is derived from a "superior" 32-column periodic table by cutting and pasting the f-block below it.
— Brian Gregory, The Global Periodic Table and a Proposed Standard Classroom Version
- Here the author is introducing/claiming a significant difference between the two forms. Even worse: for a classroom PT. Hope to to have more time for this shortly. -DePiep (talk) 15:51, 12 August 2020 (UTC)
Graphic representations etc (Mazurs 1974)
Our alternative periodic tables article refers to a work by Mazurs (1974), called "Graphic representations of the periodic system during one hundred years…". It says:
- "A 1974 review of the tables then known is considered a definitive work on the topic:[1] Mazurs, E. G. Graphical Representations of the Periodic System During One Hundred Years. Alabama; University of Alabama Press, 1974, ISBN 0-8173-3200-6."
I deleted:
- "is considered a definitive work on the topic:<ref>https://pubs.acs.org/doi/pdf/10.1021/ed052pA436.1".
Double sharp, pre-departure, reverted me, saying "(WP:OR is no good reason to delete that)".
I deleted what I did in the interests of improving WP.
Philip Stewart, a polymath who has published several articles on the PT opined that:
- "Mazurs book should be used with great care. He redrew the images, systematically adding elements that were not known when they were first published – which is acceptable – and changing their shape – which is totally unacceptable (for example figure 111 which hideously misrepresents Janet’s elegant original). Sometimes he misattributes them, for example he credits Janet with a design that is not his at all (fig. 85, which is a distorted version of Stedman’s). If he doesn’t like something he simply leaves it out, for example the element zero in tables by von Antropoff and Janet. I do not trust any illustration of Mazurs; you should always check against the original. This is unlike Van Spronsen who is scrupulously accurate.
- Mazurs book suffers also from the fact that he classifies the images in a system that is almost incomprehensible, and his bibliography and index are not cross referenced to page numbers, so that it is a heroic enterprise to find anything."
I've experienced the same thing in using Mazurs' book.
Our own article on Mazurs raises similar concerns.
I intend to re-revert Double sharp's edit on the above grounds. I won't do it for now, pending thoughts from others. Sandbh (talk) 06:36, 8 August 2020 (UTC)
- @Sandbh: After a quick examination, it seems that doi:10.1021/ed052pA436.1 only mentions "definitive review" as part of a review by George Kauffman. This would appear to be but one opinion about Mazurs' book, and not reflective of the whole community. I would not immediately re-revert, but instead amend it to accredit this statement specifically to Kauffman, and offer the counterargument as you describe with appropriate sourcing.
- For instance, change the existing phrasing to:
A 1974 review of the tables then known, considered a definitive work on the topic by American chemist George Kauffman, but considered factually inaccurate by polymath Philip Stewart. - Under NPOV, this seems like the best solution, for it gives due credit to those in support of Mazurs' book and those critical of its accuracy. However, since the article presents it in § Further Reading, it might then be more appropriate to keep the description as short and unbiased as possible, and if appropriate, describe it in greater detail in the article body. ComplexRational (talk) 17:40, 10 August 2020 (UTC)
@ComplexRational: Yes, thank you; something like that'd be the way to go. Sandbh (talk) 01:43, 11 August 2020 (UTC)
- @Sandbh: In the absence of any objections, should we proceed with this? ComplexRational (talk) 02:28, 13 August 2020 (UTC)
@ComplexRational: For sure we should. Sandbh (talk) 02:34, 13 August 2020 (UTC)
Split d-block
I stumbled upon a transcript of a 1981 Bakerian lecture, here, by RJP Williams who, with CSG Phillips, wrote a remarkable advanced two volume set on Inorganic chemistry, in 1965. From a 1967 book review: "We have inorganic chemistry presented here as the difficult and complex subject it is. The approach is thoroughly adult, and the level is probably more advanced than our students have came to expect."
Williams writes (pp. 362-363):
- 2. THE CHEMICAL CLASSIFICATION OF ELEMENTS
- "The classification of elements in the Periodic Table is now known to be a reflection of restrictions imposed by quantization of energy states of electrons in atoms. However, without recourse to other than empiricism in the study of chemistry the same classification had been observed for over 100 years. In fact it has long been a standard educational practice to separate elements into Groups IA, IIA, and IIIA; transition metals; Groups IB, IIB and IIIB; and the non-metals of Groups IVB to VIIB of the Periodic Table to simplify discussion of their chemistry. Although the distinctive properties in aqueous solution of each of the four classes does not provide sharp divisions it is very useful to treat separately three types of metal: Groups IA, IIA and IIIA metals are associated with equilibrium ionic-model chemistry; transition metals with one-electron redox chemistry and, across each such series, increasingly covalent chemistry concommitant with increasing Lewis-acid strengths of ions, usually at equilibrium with their surroundings; and Groups (IB), IIB and IIIB metal ions with a compromise ion chemistry involving strong Lewis-acid properties while maintaining fast equilibration but little redox activity…Finally, there is the further chemistry of non-metals…"
The upshot is that, according to Phillips, and in the specific context of simple chemistry, a split d block is very useful. --- Sandbh (talk) 03:28, 18 August 2020 (UTC)
Incivil behaviour by User:DePiep
Here. Sandbh (talk) 07:24, 27 September 2020 (UTC)
- ... and this is the closure: /Archive. -DePiep (talk) 20:47, 11 October 2020 (UTC)
The remarkable chemistry of the pre-halogen nonmetals
I have a completed manuscript (5,000 words) if anybody would like to review it pre-publication. --- Sandbh (talk) 00:59, 21 August 2020 (UTC)
Why do none of the transition metal groups have standardized names?
To give all eighteen groups a common name, may I propose the addition of ten more names?
1: the alkali metals (when excluding hydrogen), 2: the alkaline earth metals, 3: the disputogens, 4: the titanogens, 5: the vanadogens, 6: the chromogens, 7: the manganogens, 8: the ferrogens, 9: the cobaltogens, 10: the nickelogens, 11: the coinage metals, 12: the volatile metals, 13: the icosagens/the trigens, 14: the crystallogens/the tetragens, 15: the pnictogens, 16: the chalcogens, 17: the halogens, 18: the noble gases
We can also extend this to the f block - the lanthanogens, the cerogens, the praseodymogens, the neodymogens, the promethogens, etc.
I know this is WP:OR, but we need better names instead of trying to remember whether nickel is in group 8, group 9, or group 10. ― Дрейгорич / Dreigorich Talk 10:08, 4 August 2020 (UTC)
- @Дрейгорич: They do have standardised names, sort of, in that the IUPAC Red Book provides for them to be called according to the first element in each group, so group 3 is the scandium group.
- I’ve seen references to: early transition metals (groups 4 to 7); ferrous metals (Fe, Co, Ni); and of course the platinum group metals.
- And there is Group 5 as the acid earth metals. The name is a reference to the acidic nature of the pentoxides of this group. See: Remy H 1956, Treatise on inorganic chemistry, vol. 2, Elsevier, Amsterdam, p. 87
- --- Sandbh (talk) 11:41, 4 August 2020 (UTC)
- The acid earth metals. Huh. Well, I know what I'm naming our rock band. ― Дрейгорич / Dreigorich Talk 11:47, 4 August 2020 (UTC)
- @Дрейгорич: You'll be in good company. There was a band called Rare Earth in the 1970s. I see they're still active. Sandbh (talk) 00:35, 7 August 2020 (UTC)
- Pentoxides of V, Nb, and Ta should better be called amphoteric. Pentoxides with weak enough basic character to be safely called acidic rather occur from group 6 onwards. Double sharp (talk) 13:07, 4 August 2020 (UTC)
- Not really; it depends on the context.
- Remy writes:
- "Fifth sub-group of the periodic system: The acid earths
- Although definitely metallic in character in the elementary state, they are decidedly acidic in their normal oxides, the pentoxides. This is true of V, Nb, and Ta at least—and for this reason their pentoxides are also known as the acid earths (i.e., acid-forming metal oxides), or as the earth acids…it appears…the basic character is more strongly developed in Pa than in the first three members of the group, which are not capable of forming simple salts in aqueous solution even with the strongest acids."
- Lidin (1996), writing in "Inorganic substances handbook," refers to V2O5 as amphoteric with predominating acid properties, and Nb2O5 as an acidic oxide. Our article on the latter notes it dissolves in fused alkali. Sanderson (1967), writing in "Inorganic chemistry", rates Ta2O5 as amphoteric, favouring acidity.
- We can see then that Remy was referring to which side of the basic-acidic line the oxides fell on.
- In contrast, Group 4 is a mixed bag: TiO2 is amphoteric, favouring acidity; and ZrO2 and HfO2 are amphoteric, favouring basicity.
- I haven't heard of pentoxides of Group 6. From Greenwood & Earnshaw these seem to be intermediate compounds having formulae such as Mo4O11, Mo17O47, W18O49 and W20O58 (p. 1008). Turning now to the normal oxides of Group 6, MoO3 is "distinctly amphoteric" (Wiberg 2001, p. 1388) and dissolves in strong acids to form salts which contain the bent molybdanic ion MoO22+ in hydrated form: [MoO2(H2O)4]2+.
- Thus, Group 5 is the first group in which the normal oxides favour acidity, hence the term acid earths. --- Sandbh (talk) 01:22, 5 August 2020 (UTC)
- Greenwood and Earnshaw (2nd ed., p. 981): "V2O5 is amphoteric. It is slightly soluble in water, giving a pale yellow, acidic solution. It dissolves in acids producing salts of the pale-yellow dioxovanadium(V) ion, [VO2]+, and in alkalis producing colourless solutions which, at high pH, contain the orthovanadate ion, VO43−."
- Greenwood and Earnshaw (2nd ed., p. 982): "Niobium and tantalum also form various oxide phases but they are not so extensive or well characterized as those of vanadium. Their pentoxides are relatively much more stable and difficult to reduce. As they are attacked by conc HF and will dissolved in fused alkali, they may perhaps be described as amphoteric, but inertness is the more obvious characteristic."
- Indeed, true that there are no stoichiometric M2O5 for group 6, but we can already see acidic properties dominate more from all that polyanion chemistry famous for Mo and W. Greenwood and Earnshaw call +6 oxides of Cr, Mo, and W acidic (p. 1007).
- Of course bounds of amphotericity depend on context. There is simply a continuum from more to less acidic properties, different authors may consider a different range of what is sufficient to call an oxide amphoteric. That's why you have to pick one source's classification for consistency. Otherwise the classification is taken out of context. Nb2O5 can't possibly be more acidic than MoO3 with the lower cationic charge by standard general chemistry. Double sharp (talk) 02:37, 5 August 2020 (UTC)
Some more observations:
- Issa IM and Khalifa H 1954, The amphoteric properties of molybdenum trioxide and its isoelectric point. J. Indian. Chem. Soc. 31, 2. pp. 91-96
- Sisler HH et al. 1959, General chemistry: A systematic approach, Macmillan: "Tungsten trioxide, like its molybdenum analog, is amphoteric, with acidic properties predominating." p. 705
- Songina OA 1970, Rare metals, 3rd ed., Israel Program for Scientific Translations; [available from the U.S. Department of Commerce, Clearinghouse for Federal Scientific and Technical Information, Springfield, Va.: "converted to the anhydride MoO3 at 115 - 130°C. Both the anhydride and the acid are somewhat amphoteric and readily dissolve in solutions of alkalis." (p. 35).
[Above is an unsigned contribution by Sandbh. Double sharp (talk) 09:17, 5 August 2020 (UTC)]
Yes, no doubt you can find lots of reliable sources calling MoO3 amphoteric. But can you find any doing so that also calls Nb2O5 acidic like you do when you bring together the different bounds of amphotericity used by Remy and Wiberg? Double sharp (talk) 09:16, 5 August 2020 (UTC)
The rest of the names
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|
| Scandium group metals |
Iodide metals1 |
Acid earth metals |
Yellow earth metals2 |
Heptoxide metals3 |
Red metals4 |
Weather metals5 |
Catalytic metals6 |
Coinage metals |
Volatile metals |
| Sc Y La | Ti Zr Hr | V Nb Ta | Cr Mo W | Mn Tc Re | Fe Ru Os | Co Rh Ir | Ni Pd Pt | Cu Ag Au | Zn Cd Hg |
If we can fill out the blanks I'll see I can get the names published in a journal article. Sandbh (talk) 06:52, 5 August 2020 (UTC)
Note 1: Refers to the iodide crystal bar process: "The only metals it has been used to purify on an industrial scale are titanium, zirconium and hafnium, and in fact is still in use today on a much smaller scale for special purity needs." Sandbh (talk) 13:36, 5 August 2020 (UTC)

Note 2: The background is that chrome yellow (i.e. lead chromate) was also referred to as "orange earth". Lead chromate has an orange-yellow colour, so I can see where the reference to orange comes from. The other yellow earths are molybdenum trioxide; and canary yellow tungsten trioxide powder. Sandbh (talk) 00:19, 23 August 2020 (UTC)
Note 3: “The same probably applies to the triads of elements, between the two halves of large periods; they constitute however the transition between these two halves, between the heptavalent metals of the first half of the large periods, whose highest oxides are acidic…”, here Sandbh (talk) 10:17, 5 August 2020 (UTC)
Group 7 as the heptoxide metals refers to the observation that this is the first group with exclusively acidic oxides in their highest oxidation state. (The +6 oxides of group 6, while acidic, all show some amphoteric character.)
This is the only group in which binary metal heptoxides are found; each such oxide has a unique crystal structure (Mast 2018, p. 5).
Note 4: From the reddish-brown colour of rust; the most prevalent Ru precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically; and the red osmates OsO
4(OH)2−
2 formed upon reaction by OsO4 with a base. Sandbh (talk) 02:28, 7 August 2020 (UTC)
Note 5: From the use of cobalt chloride as a humidity indicator in weather instruments ; rhodium plating used to "protect other more vulnerable metals against weather exposure as against concentrated acids or acids fumes" (Küpfer 1954, p. 294); and the "rainbow" etymology of iridium.
Note 6: From G&E: "They are…readily obtained in finely divided forms which are catalytically very active." (p. 1148). Sandbh (talk) 07:02, 5 August 2020 (UTC)
- The new category names do not seem very effective to me. "Scandium metals" seems unnecessary when "scandium group" tells us exactly the same thing and is more standard. "Acid earth metals" for group 4 is in conflict with the citation you got for it where it means group 5 (and ZrO2 and HfO2 are rather on the basic side of amphotericity). "Aerospace metals" seems more relevant to Al than to V, Nb, and Ta. "Yellow metals" is a bit strange for Cr, Mo, and W as none of them are yellow, and Cr compounds are known for many other colours too. "Heptavalent metals" would literally include every metal that manages a +7 oxidation state. "Iron metals" will likely be confusing with the "iron triad". "Catalytic metals" would grab most of the transition metal series, not just Ni, Pd, and Pt. "Volatile metals" would literally include, say, Po.
- That is my opinion. You may disagree, and if so I will not give more comments on this since this is not WP-related. Double sharp (talk) 11:04, 5 August 2020 (UTC)
@Double sharp: Good feedback. Thanks for noting my error about the acid earths; now corrected. Group 3 are now the scandium group metals; that is better. I've reverted 6 back to ? Group 7 as heptavalent metals refers to the fact[?] that this is the first group with exclusively acidic normal oxides. I take normal here to refer to the MOS. (Aiee! There’s a blast from the past ^_^) I don’t see the term "iron triad" as often as I hear "ferromagnetic metals", and I always forget their order. I’ve changed the name to iron group metals. "Volatile metals" is in the literature for group 12; Po is a chalcogen metal. There sure is a crash in MP after group 11, as cited in our post transition metal article. I know many TM have catalytic properties. That said, if you asked me about TM catalysts, Pd and Pt would be the first to come to mind. I haven't checked G&E as to why they highlighted group 10 for their very catalytic presence.
So there are two groups to go: 6 and 9. Group 9 are the densest group of the TM. Obvious name is heavy metals but that would be confusing. Sandbh (talk) 13:22, 5 August 2020 (UTC)
- I don't think "normal" is a good characterisation of oxidation states like Cr(VI) and Mn(VII) that are well-known from high school as strong oxidants. Especially not when maximum oxidation states would include Ag(III), or, even more exotically, Ir(IX). But, since this is for outside Wikipedia, whether you agree with that or not is your choice.
- "Volatile metals" seems to have a more natural meaning of "a metal that is volatile". Polonium is one such. Using it as a group name requires disambiguating it from the natural meaning.
- Right, that's it from me. That's because I believe there is no need for special names in the first place. To me: names are for families of chemically related elements. Groups are about valence electrons and orbitals. Families are about the final result, sometimes with a dose of natural occurrence (nobody calls Lr a rare earth metal). These can be vastly different as evidenced by hydrogen in group 1 but outside alkali metals. There is no reason why they must follow especially inhomogeneous and small groups like columns in transition metals. In fact the famous family of rare earths in this region includes group 3 but expands well beyond it. So, I don't think I can give feedback about how best to do something that I don't believe is needed. You may disagree, in which case I wish you the best at it. Double sharp (talk) 13:55, 5 August 2020 (UTC)
G&E (p. 27) refer to the highest normal oxide of each element. Clearly, these are the heptoxides M2O7 for the group 7 metals. I've changed their name to the heptoxide metals.
This is for WP, as an encyclopaedia, which we always try to improve. If something we think would be an improvement is not in the literature, we can seek to get it into the literature. Example: the metalloid article was unstable due to disagreement about which elements were metalloids. I wrote an article on this which was published in JChemEd. The result? Our metalloid article is now stable and attained FA status.
I've withdrawn the name "Iron group metals" for the reason you gave i.e. that it more often refers to the ferromagnetic metals Fe Co and Ni.
"Volatile metals" is in the literature. The “volatile” refers to their low melting points compared to neighbouring metals in this part of the periodic table. Indeed, there is an abrupt and significant reduction in physical metallic character from group 11 to group 12 (Sorensen 1991, p. 3).
Special names, didactically speaking, give folks something memorable to hang their hats on. That is why they are useful. --- Sandbh (talk) 07:31, 6 August 2020 (UTC)
- @Sandbh: G&E quoting the "highest normal oxide" is not a statement about highest oxidation states always being normal. "Normal oxides" means a real oxide with O2− as the counteranion, not peroxide or superoxide. So, K2O, for instance, rather than K2O2 or KO2.
- Just getting something you think is an improvement into the literature does not mean that you can get it onto WP. If no one but you gives something in the literature, it's simply WP:UNDUE to mention it as something that looks standard on a par with group names like "halogens". See also WP:SELFCITE.
- It is increasingly my opinion that our colouring is itself not something that we should have done in the first place. Different sources show many different colourings, the colourings give the wrong idea that an element belongs to one and only one category and that they are exhaustive, and picking some sources' view as opposed to others and deciding which is better ourselves is a matter of WP:SYNTH. We can't make arguments on Wikipedia unless you can find them in the literature. It would have been better, in my opinion, to only colour blocks as I do on my userpage, as those are at least generally referred to as a basis even if few actually seem to define them. That will not necessitate any funny business with helium: it can still be an s block element above Ne. The metal-to-nonmetal trend can be mentioned, most of the text present can still be there as bald statements, but we cannot make the decision as to which to believe and colour ourselves with this much variation.
- And yes, by the way: all those arguments I have been stating in favour of Lu are fair game. They are in the literature, I did not make them up myself, so we can quote them. Double sharp (talk) 07:44, 6 August 2020 (UTC)
@Double sharp: Here's the quote by G&E: "…O elicited an increasing valence in the highest normal oxide of each element and this was directly related to group number, i.e. M2O, MO, M2O3,…M2O7." (p. 27)
I'll thank you for the courtesy of not treating me as if I were a newbie. I'm well familiar with WP:UNDUE, and with WP:SELFCITE which says, "Using material you have written or published is allowed within reason…". I'm also familiar with Wikipedia's core Ignore All Rules policy, which you recently cited. WP:SNYTH was never raised as an issue, just as we have not had to revert to WP:LAWYERING, until now. Rather, in the spirit of WP:IAR we have always sought to improve WP as an encyclopedia.
The colouring of our table is what attracted me to WP in the first place, many years ago. I suspect many other folk find it engaging. The most popular PT on the web is based on our colour-category approach. Our colouring scheme inspired my 2020 peer-reviewed article in Foundations of Chemistry, "Organising the metals and nonmetals" (2,200+ accesses to date). Among other things, the article lists several didactic advantages, including the following:
- "The accompanying periodic table (see the Electronic supplementary material) incorporates elements of learning theory with its use of annotations to facilitate perceptual grouping; colour to differentiate ideas and direct attention to key topics; and “natural" classes or clusters to organise information and help with content processing (Richey et al. 2010)."
We do not engage in the practice of, "picking some sources' view as opposed to others and deciding which is better ourselves". Rather, we strive to show what appears to be the most representative categorisation according to the literature. When a category is finely balanced, as in the roughly 50:50 treatment of group 12, we discussed it and coloured the group as PTM. When it came to astatine, R8R suggested colouring it as a metalloid, which we all supported (= no dissent).
I don't understand the basis for your statement that, "the colourings give the wrong idea that an element belongs to one and only one category and that they are exhaustive". Our PT article makes it clear this is not the case. Ditto my JChemEd article. Chemists need not lose sleep over such matters. As Nelson (2011), a chemist, said:
- "…care needs to be taken to remember that…[this classification scheme] is only an approximation, and can only be used as a rough guide to the properties of the elements. Provided that this is done, however, it constitutes a very useful classification, and although purists often despise it because of its approximate nature, the fact is that practising chemists make a great deal of use of it, if only subconsciously, in thinking of the chemistry of different elements."
It seems to me that, with occasional bouts of tethchiness, we strive (as best we can) to take account of the literature. Where the literature is shabby, we do our best to be representative, and discuss and acknowledge the shabbiness in the applicable article. Where one of us feels prompted to address the gap, and put in the sizeable effort to do so, we seek to get published in the literature.
There'll be many more arguments in support of La in group 3, when my peer-reviewed article on the location and composition of group 3 soon appears in Foundations of Chemistry (editor Eric Scerri). Sandbh (talk) 13:29, 6 August 2020 (UTC)
@Дрейгорич: That was a great question of yours. I subsequently got an e-mail from a chemistry professor saying "I love this…weather metals, especially!"
- No prob. I just had it as a random question in my head. It started with the "disputogens" and I wondered... if we're doing one group of the 18, why not all of them? ― Дрейгорич / Dreigorich Talk 03:27, 9 August 2020 (UTC)
Colleagues, here's the outcome, which I've asked Dr Mark Leach to host at the Internet database of periodic tables. Happy reading. I intend to have the names published in a peer-reviewed journal. Sandbh (talk) 02:58, 9 August 2020 (UTC)
A colleague asked why none of the transition (technicolour) metal groups have standardised names. "We need better names instead of trying to remember whether nickel is in group 8, group 9, or group 10."
They do have standardised names, sort of, in that the IUPAC Red Book (Connelly et al. 2005) provides for them to be called according to the first element in each group, so group 3 is the scandium group.
But that is a rather dull practice and I have enough trouble recalling the order of the 3d metals let alone the members of e.g. the cobalt group.
Didactically speaking, special names give folks something memorable to hang their hats on.
Here is listing of group names, including for the transition elements. Looking at the names now I recall how uninspiring my introduction was to the "no group name" transition metals, many years ago.
Group names
1 Li to Fr Hydrogen and the alkali metals 2 Be to Ra Alkaline earth metals 3 Sc Y La Scandium group metals ------------------------------------- 4 Ti Zr Hf Iodide metals 5 V Nb Ta Acid earth metals 6 Cr Mo W Chromatic earth metals 7 Mn Tc Re Heptoxide metals 8 Fe Ru Os Rubiferous metals 9 Co Rh Ir Weather metals 10 Ni Pd Pt Catalytic metals 11 Cu Ag Au Coinage metals 12 Zn Cd Hg Volatile metals 13 B to Tl Icosagens 14 C to Pb Crystallogens ----------------------------- 15 N to Bi Pnictogens 16 O to Po Chalcogens 17 F to At Halogens 18 He to Rn Noble gases
Of the above names, only those for groups 1–3 and 15–18 are mentioned in the IUPAC Red Book on the nomenclature of inorganic chemistry.
1 — Alkali metals: Rarely, hydrogen and the alkali metals. Self explanatory, but for hydrogen. Hydrogen is nearly always placed above lithium. Otherwise it is placed floating by itself (sometimes with helium) in the middle of and above the periodic table; above boron, or carbon or straddling boron and carbon; or above fluorine.
The position of hydrogen in group 1 is quite well settled. It does not matter that hydrogen is a nonmetal and a gas, in the same way that it does not matter that oxygen in group 16 is a nonmetal and a gas whereas polonium, at the other end of group 16, is a metal and a solid.
Similarities of hydrogen with lithium:
- Both have significant covalent chemistry
- Lithium, like hydrogen, when bound to highly electronegative atoms such as nitrogen, oxygen, or fluorine, is capable of forming electrostatic bonds with other nearby highly electronegative atoms
- Their spectra are similar.
Similarities with the alkali metals:
- In chemical reactions, hydrogen and the alkali metals usually lose their single valence electrons; all usually have a valence of +1
- Both form similar compounds e.g. hydrogen chloride (HCl) and alkali metal chlorides (XCl)
- Like hydrogen, most of the alkali metals can form compounds with a charge or oxidation state of –1
- Hydrogen can stand in for alkali metals in typical alkali metal structures; see: Hydrogen adopts alkali metal position (Wilson 2013)
- Hydrogen is capable of forming alloy-like hydrides, featuring metallic bonding, with some transition metals.
- The anomalous properties of hydrogen can be explained by its unique electron configuration, in that it has no underlying core of electrons.
2 — Alkaline earth metals: Historically, beryllium and magnesium were not counted as alkaline earths (Parish 1977). Beryllium is amphoteric rather than alkaline; magnesium was first isolated, in an impure form, by heating the oxide with charcoal thereby showing it was not an intractable "earth".
3 — Scandium group metals: Scandium, yttrium, and lanthanum were all discovered in mineral samples from Sweden.
There is a minor controversy as to the composition of the group. The third and fourth members are either lanthanum and actinium, or lutetium and lawrencium. Most tables show the former although there has been some increasing interest in the latter. There is an IUPAC project considering the matter, here.
4 — Iodide metals: "Van Arkel and de Boer developed the iodide decomposition process [in 1924] to make a pure, ductile metal [Zr] in Eindhoven, Holland. The "iodide crystal bar" process continues to be used today as method of purifying Ti, Zr, and Hf, even thought it is slow and expensive." (Schweitzer 2006, p. 571)
5 — Acid earths: "Although definitely metallic in character in the elementary state, they are decidedly acidic in their normal oxides, the pentoxides. This is true of V, Nb, and Ta at least—and for this reason their pentoxides are also known as the acid earths (i.e., acid-forming metal oxides), or as the earth acids…it appears…the basic character is more strongly developed in Pa than in the first three members of the group, which are not capable of forming simple salts in aqueous solution even with the strongest acids." (Remy 1956, p. 87)

6 — Chromatic earth metals: Etymology: < French chrome, < Greek χρῶμα colour.
- ^ Chromic acid, of which Cr(III) oxide is the anhydride, has an orange-red appearance
- † Cr(VI) oxide dissolves in small amounts of water to give yellow-red to red polychromic acids while in larger amounts of water it yield yellow chromic acid solution
- ‡ Reduction reactions of MoO3 in acid can yield dark red Mo2+4; green-yellow Mo+3; and yellow-orange Mo2O4+2
- § On heating, Mo or W metal is transformed progressively into violet to blue-black metallic conducting phases MO3–2
- II Tungstite WO3·H2O is formed by the weathering of tungsten containing minerals; it crystallizes in translucent yellow to yellow green masses
- ¶ Mild reduction in acid medium of WO3·2H2O gives intensely coloured tungsten blue, this being a general term applied to complex mixtures of W(VI) and W(V) oxides and hydroxides
- # "Thermochromism is the property of substances to change colour due to a change in temperature and WO3 is an attractive example for this property. On cooling the oxide by liquid nitrogen down to –196°C, a sudden change occurs from yellow to white, which then alters to a bluish-white colour between –50 and –27°C. At room temperature it becomes pale lemon-yellow again. On further heating to 200 – 300°C, WO3 becomes dark yellow, changing to a deep orange colour at 400 to 500°C” (Weil & Schubert 2013).
- ^ Chromic acid, of which Cr(III) oxide is the anhydride, has an orange-red appearance.
That chromium is the first of the chromatic earths is apt.
- Weil M & Schubert W-D 2013, "The beautiful colours of tungsten oxides", Tungsten Newsletter, June, ITAA, https://www.itia.info/assets/files/newsletters/Newsletter_2013_06.pdf, viewed Aug 5th, 2020
7 — Heptoxide metals: "The same probably applies to the triads of elements, between the two halves of large periods; they constitute however the transition between these two halves, between the heptavalent metals of the first half of the large periods, whose highest oxides are acidic…" (Walter 1909, p. 269)
Group 7 as the heptoxide metals refers to the observation that this is the first group with exclusively acidic oxides in their highest oxidation state. (The +6 oxides of group 6, while acidic, all show some amphoteric character.)
Further, this is the only group in which binary metal heptoxides are found, each such oxide having a unique crystal structure (Mast 2018, p. 5).
8 — Rubiferous metals: (classical Latin rubēre to be red; -fer producing); from the reddish-brown colour of rust; the most prevalent ruthenium precursor being ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically; and the red osmates OsO
4(OH)2−
2 formed upon reaction by osmium tetroxide with a base.
Iron causes our blood to be red. Iron is the most important structural metal. More than $10 billion is lost each year to corrosion in the US alone. Much of this corrosion is the rusting of iron and steel. The etymology of the word 'rust' has its origins in the Proto-Germanic word rusta, which translates to "redness".
That iron is the first of the Group 8 rubiferous metals is a rich juxtaposition.
9 — Weather metals: From the use of cobalt chloride as a humidity indicator in weather instruments; rhodium plating used to "protect other more vulnerable metals against weather exposure as well as against concentrated acids or acids fumes" (Küpfer 1954, p. 294); and the "rainbow" etymology of iridium.
10 — Catalytic metals: "They are…readily obtained in finely divided forms which are catalytically very active." (Greenwood & Earnshaw 2002, p. 1148).
Of course, many transition metals have catalytic properties. That said, if you asked me about transition metal catalysts, palladium and platinum would be the first to come to mind. And, per Greenwood and Earnshaw, group 10 appear to be particularly catalytic.
11 — Coinage metals: Historically, most coinage metals (or alloys) are made from copper, silver or gold, the copper usually being augmented with tin and often other metals to form bronze. Roe and Roe (1992) noted 24 metals had been used in coins of the world. Curiously, chromium and manganese were not mentioned by them, even though both elements had been used in common circulation coins (Canada wartime V nickels and US wartime Jefferson nickels, respectively) long before the time of their article's publication.
12 — Volatile metals: The "volatile" refers to their low melting points compared to neighbouring metals in this part of the periodic table. Thus, there is an abrupt and significant reduction in physical metallic character going from group 11 to group 12 (Sorensen 1991).
13 — Icosagens: A reference to the frequency of icosahedral structures seen in this group (Greenwood & Earnshaw 2002, p. 227).
14 — Crystallogens: After the capacity of carbon, silicon, germanium, and grey tin to adopt regular covalent network structures. Rarely adamantogens (Jensen n.d.) or merylides (Fernelius 1971; Fernelius et al. 1983). The origin of merylides is obscure, perhaps mer-, part; -yl, ancient Greek ὕλη (húlē, “wood, material”)" and -ide, one belonging to a specific natural group (Websters 2002), thus charcoal, coal, carbon. On this suggestion, Fernelius et al. (1971) commented that, "It has been proposed, but has not yet become widely used. Let us hope that it never does. New terms should be derived from familiar roots, not unknown ones."
15 — Pnictogens: from pnigmos, suffocation; a reference to the life-extinguishing capacity of nitrogen.
16 — Chalcogens: The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the Latinised Greek word genēs, meaning born or produced. The connection is to the ores that copper forms with sulfur, and to a lesser extent oxygen.
17 — Halogen: The name means "salt-producing". When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide.
18 — Noble gases: Rarely aerogens. Since oganesson is unlikely to be a gas and and is expected to be relatively reactive, perhaps group 18 may need to be renamed as the helium group.
- † The yellow aspect of the group 6 metals spills over into their neighbours, the heptoxide metals, and iron. Thus the heptoxides of technetium and rhenium are yellow. For manganese, on the other hand, yellow is a rare colour. There are a few references to manganese yellow glassware however the colour arises from the presence of iron rather than manganese per se. Here manganese, as green manganese oxide, oxidises iron from its ferrous (+2) state to its ferric (+3) state yielding yellow iron(III) oxyhydroxide (Corning 2011).
References
- Connelly NG, Damhus T, Hartshorn RM and Hutton AT 2005, Nomenclature of inorganic chemistry: IUPAC recommendations 2005, International Union of Pure and Applied Chemistry, RSC Publishing, Cambridge
- Corning Museum of Glass 2011, Decolorizers (Manganese dioxide)
- Fernelius WC, Loening K & Adams RM 1971, 'Names of groups and elements', Journal of Chemical Education, vol. 48, no. 11, p. 730; Fernelius WC 1983, 'Group names', Journal of Chemical Education, vol. 60, no. 2, p. 140.
- Greenwood NN & Earnshaw A 2002, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford
- Grochala W 2020, Watch the colors: or about qualitative thinking in chemistry, open access
- Jensen WB n.d., The periodic law and table, unpublished article for Encyclopedia Britannica, p. 10
- Küpfer YJ 1954, Rhodium uses in plating, Microtecnic, Agifa S.A.
- Mast, DS 2018, Crystallographic exploration of fundamental technetium species at nonambient conditions, UNLV Theses, Dissertations, Professional Papers, and Capstones 3368
- Parish RV 1977, The metallic elements, Longman, New York
- Remy H 1956, Treatise on inorganic chemistry, vol. 2, Elsevier, Amsterdam
- Roe J and Roe M 1992, "World's coinage uses 24 chemical elements", World Coin News, Feb. 17, pp. 24–25; Mar. 2, pp. 18–19.
- Schweitzer PA 2006, Fundamentals of metallic corrosion: Atmospheric and media corrosion of metals, 2nd ed. CRC Press, Boca Raton, p. 571
- Sorensen EMB 1991, Metal poisoning in fish, CRC Press, Boca Raton, Florida, p. 3
- Walter CW (ed) 1909, Ion, vol. 1: Zeitschrift für Electronik, Atomistik, Ionologie, Radioactivität und Raumchemie, London
- Websters 2002, A dictionary of prefixes, suffixes, and combining forms
- Wilson P 2013, Hydrogen adopts alkali metal position, Chemistry World
--- Sandbh (talk) 02:58, 9 August 2020 (UTC)
From symmetry-regularity to asymmetry-irregularity: The texture of the world
| Tier | Notes |
|---|---|
| 1a | Protons = regular = LSPT triads' 2nd/3rd members occur in periods of equal lengths^ |
| 1b | Electrons = regular arrangement = Madelung rule regular electron configurations |
…via symmetry breaking
| Tier | Detail | Boundary |
|---|---|---|
| 2 | Electrons = ~20 imperfections | Madelung rule snap-back† |
| 3 | Neutrons = 254 stable isotopes | Neutron: proton ratio ≤ phi (golden ratio) |
| 4 | Elements = many irregularities | Hsueh & Chiang (1937); -AR-EA-IE-EN- ring |
| 5 | Periodic systems = 1,000+ | Physics—Chemistry × Didactic—Designer matrix |
^ see doi:10.1002/chem.201900460. Fig. 8
† after each variation from the MR the filling sequence returns to normal
- Table updated Sandbh (talk) 12:21, 23 August 2020 (UTC)
--- Sandbh (talk) 07:31, 22 July 2020 (UTC)
- If you cannot make a legible statement, Sandbh, then remove this post yourself please. -DePiep (talk) 00:24, 25 July 2020 (UTC)
Regularity and symmetry
It's curious that interest in the regularity and symmetry of periodic system representations has some basis in Z and the Madelung Rule. Yet the MR sort of has some bumps in it, and there was the early crisis of confidence in the periodic table when the old school physicists and chemists had to grapple with what to do with isotopes of the elements. Having accommodated that, it turns out the periodic law is only an approximation, rather than a Law, as such. That leads into the observation that there is no ideal periodic table, since the table depends on the properties of interest. Hence Tier 5. From the perfection of Tier 1, we descend into the "chaos" of Tier 5.
Thus, an emerging field of thought is the importance of symmetry breaking, rather than pure symmetry:
- 1. "…symmetries matter, largely because we like to see them broken sometimes: the laws, particles and forces of physics all have their roots in symmetry-breaking. They create what David Gross of the Kavli Institute for Theoretical Physics at the University of California, Santa Barbara, calls the "texture of the world". These considerations have led Florian Goertz at the Max Planck Institute for Particle and Astroparticle Physics in Heidelberg to propose the existence of a new particle that is single-handedly capable of cleaning up five of the stickiest problems in physics. "Complete symmetry is boring," says Goertz. "If symmetry is slightly broken, interesting things can happen." " (Brooks 2018, p. 30)
- 2. "Through the work of many physicists, the concept of broken symmetry was introduced into elementary particle physics in the 1960s and 1970s. The idea was, in the simplest language, to keep the mathematical forms symmetrical, but the physical consequence unsymmetrical. The standard model, for which Glashow, Salam, and Weinberg shared the Nobel prize in 1979, was based on gauge theory with broken symmetry. It has been extremely successful." (Yang 1996, p. 286)
- 3. "Physical chemistry is fundamentally asymmetric. How could it not be when the proton weighs so much more than the electron?" (Philip Stewart, pers. comm. 30 Dec 2019).
- Evidence of new physics could have been under our noses all along
- 4. "Many of these remaining problems boil down to one. Crudely phrased, some things are exceptionally small while related things are exceptionally big. This is known as the hierarchy problem, and once you spot it, you start seeing it everywhere.
- Take the four fundamental forces of nature. The weakest two are gravity, and the weak nuclear force, which only operates on the tiniest of scales and is responsible for certain types of radioactive decay. The weak force is weak, but compared with it, gravity is some 25 orders of magnitude weaker – a bizarre state of affairs that, as yet, has no good explanation.
- The asymmetry reappears elsewhere. Dark energy, the mysterious force that is causing the universe’s expansion to accelerate, is 120 orders of magnitude weaker than we would expect. Dark matter, which is the dominant form of matter in the universe, interacts very weakly with regular matter. Neutrinos, the lightest particles in the standard model, are thousands of times lighter than anything else.
- These disparities are profoundly vexing to physicists, who prefer to see related parameters in a theory take broadly consistent values. This preference for "naturalness" drives much theoretical speculation – some would say to a fault. "Nature doesn’t care about our aesthetics," says [Nathaniel] Craig [a theoretical physicist at the University of California, Santa Barbara].
- Ten years on, nothing has changed. We were fixated on supersymmetry for too long, says Isabel Garcia at the University of California, Santa Barbara, searching under the convenient street light to the detriment of the field. But the story of the LHC is far from over. The collider has recorded only 3 per cent of the data we expect it to collect in its lifetime, and an upgrade to higher energies in 2020 will further raise its chances of seeing something surprising.
- But the LHC's failure to break any new ground has emboldened a new generation to question the hunches that motivated previous searches. "This optimism is most widespread amongst the youth," says Matthew McCullough, a theoretical physicist at CERN. "We've shaken off the cobwebs of the theories handed down by our PhD advisers." " (Eure 2019)
It remains to be seen if the YAPs (young asymmetrical pups) can teach the OSDs (old symmetrical dogs) some new tricks
Even so I consider that (a) asymmetry cannot be appreciated or understood without understanding (b) symmetry, and how and why things go from (b) to (a). See also Hegstrom & Kondepudi (1990), and Rosen (1996).
- Brooks, M.: This one particle could solve five mega-mysteries of physics. New Scientist, 3191, (2018)
- Eure, J. Evidence of new physics could have been under our noses all along. New Scientist, 3217, 16 Feb (2019)
- Hegstrom, R.A., Kondepudi, D.K.: The handedness of the universe. Sci. Am. 62, 108–115 (1990)
- Rosen, J.: Symmetry in science: An introduction to the general theory. Springer, New York (1996)
- Yang, C.N.: Symmetry and physics. Proceedings of the American Philosophical Society. 140, 267–288 (1996)
--- Sandbh (talk) 02:19, 25 July 2020 (UTC)
Four irregularities
Each of the four irregularities namely electrons; neutrons; element properties; and periodic systems has some kind of guided boundary.
This reinforces my impression that all periodic systems have their place under the sun. That is the key learning for students.
Null sub-section
Tier 2: Electrons
The Madelung Rule always returns to running true after each wobble (analogous to a gyroscope?).
Tier 3: Neutrons
Their numbers are bounded by the neutron drip line, a subject about which, as yet, I know little.
Boeyens and Levenids (2008, p. 144) write:
- "Harkins (1931) discovered a classification of the the stable nuclides in terms of the ratio N/P and showed that this ratio never exceeds the value of 0.62 in atomic species. The same classification was rediscovered independently many years later (Boeyens 2003) and the maximum was shown, more precisely, to be the golden ratio = 0.6180."
- Boeyens, JCA 2003, J. Radioanal. Nucl. Chem., 257, 33
- Boeyens JCA and Levendis DC 2008, Number theory and the periodicity of matter, Springer
- Harkins 1931, Phys. Rev., 38, 1270
Tier 4: Element irregularities
The irregularities in the properties of the elements might possibly be encompassed by the work of Hsueh and Chiang (1937). See appendix 2.
Chemically, the metallic or nonmetallic nature of the elements correlates with, to at least a first order approximation, the four key properties of
- atomic radius
- electron affinity
- ionization energy; and
- electronegativity.
On atomic radius, Peter Atkins (2019) wrote:
- "The periodic table and the concept of the elements of education inspires all manner of other thoughts. One is the desert-island thought: if you were asked to identify the central elemental concept summarized by the periodic table which, with you isolated on a conceptual desert island and asked to set about rationalizing chemistry, what would it be? My choice would be atomic radius."
Godovikov & Hariya (1987) showed a relationship between atomic (orbital) radius and electron affinity:
Myers (1981) demonstrated a smooth curvilinear relation, for vertical groups in the periodic table, between the electron affinity of an atom and the ionization energy of its neighbour with atomic number larger by one:
I showed a correlation between EN and radius in my Constellation of electronegativity:
Thus:
Atomic
radius→ Electron
affinity↑ ↓ Electro-
negativity← Ionization
energy
Tier 5: Periodic systems
I hope my periodic system landscape, based on the ideas of Scerri and Schwarz, represents a crude start to showing some semblance of order.
Appendix: Symmetry breaking
Much like a pencil falling to the ground from its tip in a trade-off of symmetry for stability, Davies (2007) writes that the Big Bang could have established a complex but stable universe (or multiverse) from symmetry breaking as the heat radiation in "space" lowered abruptly past the Curie Point.
Ethan Siegel, an astrophysicist, concludes:
- "Our Universe may not be as elegant as we hoped for after all."
That said, there is a lot of order amongst the disorder. That seems to be the key: pure order = complete stasis; pure disorder = complete chaos. Interesting things happen between the two poles. As the Goldilocks principle goes, "Neither too much nor too little, but just right."
- "When Coleridge tried to define beauty, he returned always to one deep thought; beauty, he said, is unity in variety! Science is nothing else than the search to discover unity in the wild variety of nature,—or, more exactly, in the variety of our experience. Poetry, painting, the arts are the same search, in Coleridge’s phrase, for unity in variety."
- Bronowski J 2011, Science and human values, Faber & Faber, London
Appendix: Chin-Fang Hsueh and Ming-Chien Chiang
J. Chinese Chem. Soc, 5, 263 (1937). (In English)
I attempted to get a copy of this article via the Australian National Library, and the British Library Lending Service, without success. The Australian National University holds copies of the serial involved, but this particular volume was missing.
The article is cited in Moeller’s Inorganic chemistry (1953, p. 119) in the following passage:
- "In an extremely interesting and searching article, Hsueh and Chiang consider any periodic property to consist of two factors, one a periodic factor determining the periodicity and the other an amplitude factor causing numerical change in the property within a given family of elements.
- The periodicity factor is, in turn, a function of valency or outermost electronic configuration, and the amplitude factor is a function of energy state and atomic radius.
- The periodicity function may be either a maximum at the center of a period or a minimum at the center.
- Periodic properties of the increasing class embrace atomic frequency, melting point, boiling point, etc., whereas those of the decreasing class are atomic volume, atomic radius, atomic parachor, etc.
- Correspondingly, the amplitude function may amount to either parallel or crossing combination, that is, the amplitudes for the sixteen periodic families may simultaneously increase or decrease or they may change in reverse order for positive and negative elements.
- Properties involving parallel combination are such ones as atomic volume, atomic radius, ionic radius, and ionization potentials, whereas those involving crossing combination are such ones as melting point, boiling point, and hardness.
- Periodic properties may therefore be classified, according to Hsueh and Chiang, into the four general types: parallel amplitude, increasing periodicity; parallel amplitude, decreasing periodicity; crossing amplitude, increasing periodicity; and crossing amplitude, decreasing periodicity.
- By combining periodicity and amplitude functions, Hsueh and Chiang derive a property equation [to follow, Sandbh] from which the numerical magnitude of a property P is related to the atomic number Z of the element in question in terms of valence V, a function of the periodic factor y, the principal quantum number n, and two parameters a and p, which are constants for a given family of elements but different for different families.
- By means of this equation and a consideration of the types of periodic properties already mentioned, theoretical variations in properties are evaluated and found to be in reasonably good agreement with observed variations. Certainly curves plotted from theoretical values so calculated against atomic number agree closely with similar ones drawn from measured values."
--- Sandbh (talk) 07:45, 26 July 2020 (UTC)
Note re activity
Weekend is over for me, so just a heads up that I will almost certainly not be able to post walls of text and do significant article redrafting work on the weekdays. But I will look in and try to comment when pinged. On weekends – possible frenzied activity again to resolve these conversations I hope. ^_^ Double sharp (talk) 00:10, 19 October 2020 (UTC)
A small question
@EdChem and Double sharp: Do you think it would be helpful to move EC's comment and DS's reply from WT:ELEM § Opinions to WT:ELEM § Supplements? It would seem that it fits better in that section. Give me your answer here and if both of you approve, one of you can move it to the most logical place, adding a small note about the move. YBG (talk) 05:49, 19 October 2020 (UTC)
- @YBG: I'm fine with it, myself. Double sharp (talk) 08:58, 19 October 2020 (UTC)
- No objection, thanks for asking. :) EdChem (talk) 00:11, 20 October 2020 (UTC)
- OK, I have moved it to § Moved from Opinions to Supplements. YBG (talk) 02:52, 21 October 2020 (UTC)
