Wikipedia talk:WikiProject Elements/Archive 48
| This is an archive of past discussions on Wikipedia:WikiProject Elements. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 45 | Archive 46 | Archive 47 | Archive 48 | Archive 49 | Archive 50 | → | Archive 55 |
The talk page is in orbit again.
This is the point where I'd calculate how many atoms tall the talk page is, but I'd better not. ― Дрейгорич / Dreigorich Talk 06:14, 11 July 2020 (UTC)
- Theorem. It is impossible to complain about the length of a talk page on the talk page without adding to the problem.
- Proof. Exercise.
- ^_^Double sharp (talk) 10:17, 11 July 2020 (UTC)
Melting points group 3
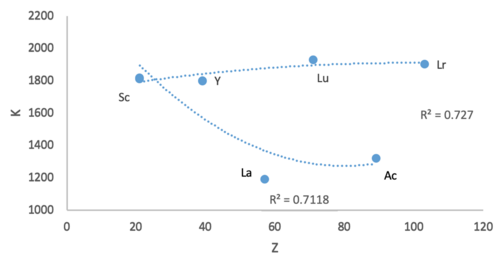
@Double sharp: In this plot the trend line curves for the two options have the same goodness of fit values. Yet, when I look at the La curve, I see that Y and La are further away (in opposite directions). Is there term for referring to this e.g. Y and La show greater bias, variance, or displacement? Sandbh (talk) 00:10, 19 June 2020 (UTC)
- @Sandbh: Variance is for the underlying distribution, so maybe better try Residual sum of squares and Residual standard error. Notice how Sc-Y-Lu makes a better trend.
- This is precisely one of the reasons why I find Sc-Y-La a bad option. Lanthanum and actinium, if placed in the d-block, always significantly increase the range of variability of the block's elements. No d-block element is as hard, as big, as electropositive, as reactive, by a significant margin. In the f-block they don't do that. Double sharp (talk) 02:20, 19 June 2020 (UTC)
@Double sharp: Yes, I agree Sc-Y-Lu makes a better trend. Is it accurate to say that while the fit values are comparable, Y and La show more variance from the Sc-Ac trend line? Sandbh (talk) 07:28, 19 June 2020 (UTC)
- @Sandbh: I would rather talk about the residual sum of squares. Variance would refer to deviation from the average of the predicted values. Double sharp (talk) 07:32, 19 June 2020 (UTC)
@Double sharp: I'll say, "While the fit values for the two options are comparable, Lu-Lr is preferred since Y and La show a greater departure from trend." Sandbh (talk) 07:57, 19 June 2020 (UTC)
- @Sandbh: Yeah, that should be fine. Double sharp (talk) 08:05, 19 June 2020 (UTC)
Landau & Ligshitz (1958): Redux

A puzzling layout
The authors discuss aspects of the periodic system of D I Mendeleev. The electron configurations of H and He are briefly noted. This is followed by three tables setting out the electron configurations of the sp, d and f elements. I’ve joined up their note and three tables to produce the subject periodic table. Curium was the last known element at their time of writing; transcurium elements are shown in parentheses.
Some extracts from their discussion follow:
- "The elucidation of the nature of the periodic variation of properties, observed in the series of elements when they are placed in order of increasing atomic number, requires an examination of the peculiarities in the successive completion of the electron shells of atoms. (p. 252)
- Many properties of atoms (including the chemical properties of elements…depend principally on the outer regions of the electron envelopes.
- The elements containing complete d and f shells (or not containing these shells at all) are called elements of the principal groups; those in which the filling up of these states is actually in progress are called elements of the intermediate groups. These groups of elements are conveniently considered separately. (p. 254)
- We see that the occupation of different states occurs very regularly in the series of elements of the principal groups : first the s states and then the p states are occupied for each principal quantum number n. The electron configurations of the ions of these elements are also regular (until electrons from the d and f shells are removed in the ionisation): each ion has the configuration corresponding to the preceding atom. Thus, the Mg+ ion has the configuration of the sodium atom, and the Mg++ ion that of neon. (p. 255)
- Let us now turn to the elements of the intermediate groups. The filling up of the 3d, 4d, and 5d shells takes place in groups of elements called respectively the iron group, the palladium group and the platinum group. Table 4 gives those electron configurations and terms of the atoms in these groups that are known from experimental spectroscopic data. As is seen from this table, the d shells are filled up with considerably less regularity than the s and p shells in the atoms of elements of the principal groups. Here a characteristic feature is the "competition" between the s and d states.
- This lack of regularity is observed in the terms of ions also: the electron configurations of the ions do not usually agree with those of the preceding atoms. For instance, the V+ ion has the configuration 3d4 (and not 3d24s2 like titanium) ; the Fe+ ion has 3d64s1 (instead of 3d54s2 as in manganese).
- A similar situation occurs in the filling up of the 4f shell ; this takes place in the series of elements known as the rare earths.† The filling up of the 4f shell also occurs in a slightly irregular manner characterised by the "competition" between 4f, 5d and 6s states.
- † In books on chemistry, lutetium is also usually placed with the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group.
- The last group of intermediate elements begins with actinium. In this group the 6d and 5f shells are filled, similarly to what happens in the group of rare-earth elements. (pp. 256–257)"
Observations
The table that arises from merging their three sub-tables is 16- rather than 18-elements wide. It may be the squarest useful table I’ve seen (16w x 15h).
L&L are sometimes cited as providing the earliest argument for placing lutetium in group 3.
Looking at their tables of electron configurations, and their categorisation of principle and intermediate elements, I suggest this is a misinterpretation of their position. A more plausible interpretation is that they supported lanthanum and lutetium in group 3, an option which other authors have featured from time to time. The earliest example I've seen is that of Bohr (1922), which features bifurcations at Na, Mg, and Y but no group numbers, per se.

A more recent example is that of Silberberg (2006).
I remember some discussion about placing lawrencium 7s27p1 under thallium 6s26p1. This was discounted since the position under thallium was already occupied by nihonium. It works fine however within L&L's paradigm.
The puzzle
The authors exclude lanthanum from the rare earths since the 4f shell has not started filling. Yet actinium and thorium are included by them with what we now call the actinoids even though these two metals have no f electrons (p. 258).
No explanation is provided for this puzzling lack of consistency with their categories. In this light I’ve moved actinium and thorium out of the actinoids and into the d-block. Sandbh (talk) 02:26, 28 June 2020 (UTC)
- @Sandbh: Thank you for pointing out the "puzzling lack of consistency" regarding thorium, with no f electrons in the gas-phase ground state but placed in the f block. While lanthanum, in the exact same situation, is denied f block membership. This is, in fact, an inconsistency that is inherent in the La table as soon as you claim that it is based on ground-state electron configurations. Not to mention the inconsistency in which lutetium and lawrencium with no valence f electrons get to appear in the f block. I have been saying this for the last few months already, of course.
- The most chemically reasonable way to deal with this, of course, is to note that the "irregular manner" in which the d and f shells fill up is a sign that the d shell is very close in energy to the s shell. Same for the f shell. If you look at how low-lying the excited states where the occupancy is changed you will realise that there isn't any sense in saying "this is the ground state and nothing else is important" because the excitation energies are well within reach of chemical bonds. This neatly avoids the contradiction by treating all the elements I mentioned above in the same way. Lanthanum, actinium, and thorium all have readily accessible f orbitals populated in chemistry and go in the f block. Lutetium and lawrencium are denied membership because their f electrons don't contribute to bonding MOs, so they go to the d block. Zinc, cadmium, and mercury can however stay in the d block because their d electrons can contribute to bonding MOs (even though they are not known in states above +2). Only the s block requires any supplementary explanation, as do the superheavies.
- The best thing about this approach, of course, is that it immediately clamps down on any desires to reflect lawrencium's odd anomalous ground-state configuration to duplicate it over thallium. Which is not discounted because nihonium already occupies that position, but first and foremost because lawrencium often has 6d1 in compounds anyway, and Lr has a lot more to do with Lu than Tl chemically and physically. The anomaly means nothing for almost all of chemistry. Just like the anomaly in which chromium and molybdenum have d5s unlike tungsten d4s2. Double sharp (talk) 03:36, 28 June 2020 (UTC)
The PT and the physics that drives it
Here. By Schwerdtfeger, Smits & Pyykkö (2020).
Some passages of interest:
- "The periodic table can be seen as parallel to the Standard Model in particle physics, in which the elementary particles known today can be ordered according to their intrinsic properties. The underlying fundamental theory to describe the interactions between particles comes from quantum theory or, more specifically, from quantum field theory and its inherent symmetries."
- "…the important Aufbau principle introduced by Bohr and Pauli that, together with Hund’s rule, is considered as the second building block of the PTE, after the atomic-number ordering. Chemical behaviour is the third most important criterion that guides the order of elements in the PTE and an essential tool for all chemists."
That's interesting. So the three building blocks are held to be: [1] Z ordering; [2] Aufbau principle + Hund’s rule; [3] chemical behaviour.
- "Despite the huge success of the Madelung–Janet rule, the most appropriate definition of the start and end points of the lanthanide and actinide series remains a matter of dispute. Inserting the lanthanides La–Yb and actinides Ac–No between groups 2 and 3, and Lu [4f145d16s2] and Lr [5f147p17s2] (note the difference in the occupation of p and d levels between the two elements) into group 3 fulfils the Madelung– Janet rule and results in a more natural placement of these elements into the PTE. However, placing La [5d16s2] and Ac [6d17s2] into group 3 and the series Ce–Lu and Th–Lr afterwards has the advantage of keeping La and Ac as the first elements of the lanthanide and actinide series to which they give their names. In a set of molecules, Xu and Pyykkö found that Lu and Lr behave in a very similar way. Moreover, the placement of the 4f-to-6f and the 5g elements in Fig. 1c keeps the group number, G, equal to the number of valence electrons. We are not delving further into discussions of chemical similarities between the two different definitions of the group 3 elements, as there are many different opinions on this. The International Union of Pure and Applied Chemistry (IUPAC) conveniently avoids this controversy by leaving the two positions in periods 6 and 7 of group 3 empty and listing 15 instead of 14 elements for the lanthanides and actinides, thus counting from f 0 to f 14.
- "1s elements. We start our discussion by mentioning the two most abundant elements in our universe, H and He, synthesized directly in the primordial nucleosynthesis roughly 10 seconds to 20 minutes after the Big Bang. These are placed into groups 1 and 18, respectively, although their chemical and physical behaviour is quite distinct compared with their heavier homologues in the PTE."
With regard to hydrogen I suggest its distinctiveness compared to the alkali metals is over-emphasised.
- "He fits rather into group 18 than into group 2 of the PTE, although we note the existence of gas-phase cations, such as HeH+, or metal helides, such as VHe3+, YHe3+ or AlHe3+ (refs 53–55), and the observed high-pressure electride compound Na2He. This is a prime example in which chemical similarity wins over electron configuration."
- "Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind."
- "HgF4 has been identified not too long ago by Wang et al."
As we know, experiments conducted in 2008 could not replicate HgF4. That said, it has been predicted to be stable at high pressure, within the range of 30 to 40 GPa, or so.
- "Stability of superheavy elements The heaviest naturally occurring elements of the PTE on our planet are U and trace amounts of 244Pu found in the deep sea floor. In fact, until 1943, only the elements up to Pu, which was produced by a deuteron bombardment of 238-U by Seaborg and colleages, were known (Fig. 1b). At that time, names like 'ultimium' or 'extremium' were considered for Pu because of the erroneous belief that this element might be the heaviest possible in the PTE"
- "We can expect new elements, perhaps up to nuclear charge 126, in the next decade or so."
- "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE."
There is a reference here to an interesting article by Restrepo on "Challenges for the periodic systems of elements: Chemical, historical and mathematical perspectives". Sandbh (talk) 01:31, 7 July 2020 (UTC)
- @Sandbh: Funnily enough, if you subscribe to those three building blocks in order, a Sc-Y-Lu table is the result. Because the chemical differences between La and Lu are certainly not so great as to justify rupturing the Aufbau-mandated d-block. As a matter of fact they are absolutely tiny. The one chemical difference that almost everyone agrees is worth reflecting, even though it breaks a rectangle, is helium. And surely nobody seriously believes that the chemical difference between lutetium and yttrium (which should rather be called an expected lack of differences, beautifully matching Zr/Hf and Nb/Ta) is of the same order of magnitude as that between helium and beryllium. Not to mention that the opinion that even this difference should not be reflected is gaining ground (the He-Be table). And also not to mention that putting helium in group 18 doesn't really change its block assignment: we still know it's a weird s-block element. Meanwhile, dumping La in group 3 changes the block assignment of La, Ac, Lu, and Lr in a silly way. Because lutetium and lawrencium are such terrible f-block elements that they don't even at least weakly use the f orbitals the way Zn, Cd, Hg do their d-orbitals. Instead all they do is use d-orbitals like scandium and yttrium. And lanthanum and actinium somehow cannot resist hybridising with f orbitals in the same way cerium and thorium do.
- And, of course, the chemical differences, if you do analyse them, all corroborate those electronic differences and point to Lu rather than La being a better fit for the d-block. Lutetium is much more like a transition metal than lanthanum, whereas lanthanum makes a perfectly normal early f block element with its superbly high coordination numbers and cubic molecular geometries betraying the use of f orbitals. For actinium and lawrencium the difference becomes even bigger in favour of Lr in the d-block.
- The group number only fits the number of valence electrons in a Lu table. Lutetium and lawrencium have only three valence electrons, the 4f and 5f shells are buried and inaccessible for chemistry. Ergo, you get an unnecessary irregularity counting valence electrons in a La table (4, 5, 6, 7, ..., 16, 3), but not in a Lu table (3, 4, 5, 6, ..., 16).
- Additionally, it is actually even more clear that La is the first lanthanide and Ac is the first actinide if you put it together with the rest of them. Otherwise it looks like La isn't a lanthanide and Ac isn't an actinide. It is much more reasonable to do that to Lu and Lr, because especially Lr is a very poor fit for the late actinide trend in its trivalency to the exclusion of any other oxidation state, while Cf through No are steadily increasing the stability of the +2 state as you go along. Not to mention that "lanthanide" as it stands is a somewhat silly category because it is trying to both be a "chemistry" category and a "physics" category. It panders to physics by excluding yttrium in the 4d row (which is nevertheless like an Ln in pretty much every way chemically), but panders to chemistry by adding lutetium in the 5d row (which is not a 4f element). There's a reason why most general-chemistry books about the lanthanides include yttrium and often scandium as well: the "rare earth metals" category is a significant improvement. Of course it's not a perfect improvement, because actinium and lawrencium are not usually included. But whoever decreed that chemical categories had to be neat and follow groups? That's where all the bad predictions of astatine come from.
- Meanwhile, it doesn't really matter that HgF4 has not been found yet. The chemical bonding in ZnF2 already significantly involves the Zn 3d MO, so it's more or less a given that d orbitals are necessary to understand group 12 chemistry. Of course for mercury they are more destabilised and it should be even more easy to see them at least with such an effect.
- "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE." Indeed! One can go back and forth with them and throw out lots of denizens of Pandora's box like Be-Mg-Zn, B-Al-Sc, C-Si-Ti, Ti-Zr-Ce-Th, Ti-Zr-Hf-Th, Sc-Y-La-Ac, V-Nb-Ta-Pa, Cr-Mo-W-U, Ca-Sr-Yb, H-F-Cl, H-C-Si, H-B-Al, etc. etc. etc. With just chemical arguments going back and forth you'll never get rid of all of them. More likely you'll end up disposing of one of them with arguments that perfectly support another that you don't want. That's why it's so much better to stick to blocks alone. They are more consistent. Even with the helium-over-neon exception they still beat everybody else because no one can seriously claim that any other element in the "pure blocks" scheme would look as chemically misplaced as helium over beryllium (second place would be hydrogen over lithium, which is why even a floating H is somewhat consistent), and so a certain level of consistency is retained even though putting a bar in the first place is a little bit arbitrary.
- I subscribe to the prediction of E126 by 2030. ^_^ Double sharp (talk) 03:46, 7 July 2020 (UTC)
Rupturing the d-block. There is no fundamental imperative either way for splitting or keeping the d-block together. Not to mention there is no issue in the 18-column form. An analogous uneven distribution occurs with Groups 1−2, and 12−18, which become spatially separated by Groups 3−11, although this is not as extreme. Here, Groups 1−2 and 12−18, from a chemical point of view, effectively form a joint “sp-” block of elements.
As Imyanitov (2015 pp. 153–154) observed:
- "If one seeks for the maximum chemical utility…[one] should opt for the more ‘unruly’ tables. If one seeks maximum elegance and orderliness above all…[one] should favor the more regular representations."
- Imyanitov N.S.: Spiral as the fundamental graphic representation of the Periodic Law. Blocks of elements as the autonomic parts of the Periodic System. Found. Chem. 18, 153–173 (2016)
Likewise, Eugen Schwarz (2019, pers. comm., 8 Dec) commented, "The real, rich pattern of elements’ chemistry does not fit into a clear-cut rectangular grid."
A high degree of orderliness, and explanatory power, can nevertheless be found in Rossotti's (1998) split d-block periodic table template. Rossotti shows where each sub-shell starts; how the lanthanoids and actinoids are inter-positioned between Groups 2 and 4, and the electron configuration make-up of each individual element and its predecessor. Here, the lanthanoids run from cerium to lutetium; and the actinoids from thorium to lawrencium. The split d-block is thus integrated into the overall design of the table.
Chemical differences. Saying, “As a matter of fact they are absolutely tiny” is hard to digest. Frex, there is nearly a 20% difference in ionic radius. Whereas La is a strong base with a knight’s move relationship to pre-transition metal Ca, Lu is amphoteric (as I recall you noted), with a weak (n) (n +10) relationship to post-transition metal Tl, also amphoteric, on the other side of the ditch.
He-Be. On this configuration gaining ground, that ignores what the authors said i.e., "…almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind." One of the referees for my Group 3 article observed that "He over Be requires a lot humour." As Scerri opined, "...it helps to remember that, when all is said and done, the periodic table remains primarily in the domain of chemistry…".
Lu-Lr. “Because lutetium and lawrencium are such terrible f-block elements…” is equally unpalatable. If that was really the case they would’ve long ago been booted out of the f-block.
Group # fit. I noticed their Fig. 1c where they line up the fifteen Ln so the number of fds-electrons lines up with groups 3 to 17. La with d1s2 lines up under Group 3, Ce with f1d1s2 lines up under Group 4 through to Yb 4f146s2 with sixteen electrons lining up under group sixteen and Lu 4f145d16s2 with seventeen under group 17. This does not work so well if Lu is placed under Y since the counting rules for Lu-Lr are suddenly changed from 17 to 3, whereas it works both ways for La-Ac (they are always 3). That is another example of Lu-Lr letting the team down, so to speak.
Not forgetting, with La in Group 3, the number of f-electrons in the trivalent cations of the Ln corresponds perfectly with their position in that block. As noted in the "physics that drives it" article:
- "Unlike the d- block elements, the compact 4f electrons are little involved in chemical bonding and act as 'spectators'. The 4f electrons can, thus, be treated (to a certain extent) as core-like. The formation of chemical bonds between Ln and other elements mainly involves Ln 6s and 5d. Note that it is not important if the 5d shell is occupied in the atomic ground state, as long as it is energetically available. This is the main reason for the chemical similarity of the lanthanides — many of us know how hard hard it is to separate the different lanthanides."
Lr as a very poor fit. This is not a showstopper. The same could be said of Sc and Zn and their distinctive failure to show typical transition metal properties. (As you said, "…whoever decreed that chemical categories had to be neat and follow groups?")
Lanthanide a somewhat silly category. No doubt that is why it is in such widespread use. It is a fine category for referring to a set of elements the properties of which are associated with (a) the progressive filling of the 4f sub-shell, thereby showing (b) a distinctive successive contraction in ionic radii, and (c) double periodicity. Yttrium, a quasi-lanthanide, does not meet any of these criteria. Lanthanum does not meet these criteria. You can shoehorn lanthanum into (c) but I have argued and maintain that Lu does a better job in that respect. Conflating Y with the Ln obfuscates the distinctive properties of Y e.g. "The fluoride complexation behavior of yttrium differs distinctly from the behavior of any rare earth" doi:10.1023/A:1005186932126; "Yttrium differs somewhat from other rare-earth elements in its spectroscopic and complex-forming properties and thus can be determined by selective methods", here;; "According to the data of [62], the nitride of yttrium differs from the nitrides of the other lanthanides in its negative temperature coefficient of the electrical resistance, which is characteristic of semiconductors, here.
La 1st Ln; Ac 1st An. I agree with the authors: La is not a lanthanoid; Ac is not an actinoid. Rather, they are progenitors of the Ln/An; pre f-block elements if you will.
Fuzziness = unnecessary disputes. I agree with the unnecessary part, as in your examples of Be-Mg-Zn, B-Al-Sc, C-Si-Ti, Ti-Zr-Ce-Th, Ti-Zr-Hf-Th, Sc-Y-La-Ac, V-Nb-Ta-Pa, Cr-Mo-W-U, Ca-Sr-Yb, H-F-Cl, H-C-Si, H-B-Al. I agree with the approach taken for H and He i.e.:
- "Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind." [italics added]. So we show Be and Mg in group 2, keeping in mind their close familial relationship with Group 12."
On the other hand, La-Lu is an argument worth having in the context of Scerri’s not unreasonable request for one element, one place.
Sticking with blocks alone. That is what most chemistry text-book authors do. They then drill down into the electronic filling sequence, and present the table as Sc-Y-La because it’s not until Ce and Th where f- electrons first make their presence felt. (An objection can be raised to Th d2s2. Still, the presence of ~0.5 of an f-electron is indicated in the solid). And we know the split d-block doesn't become over-visible due to the predominance of the 18-column form.
Restrepo reckons periodic systems, "are the interweaving of order and similarity relationships of the chemical elements".
As I have argued, and maintain, Lu in Group 3 unnecessarily disrupts this rich tapestry of chemical relationships. Sandbh (talk) 06:12, 8 July 2020 (UTC)
Long reply
@Sandbh: I apologise for the length of this. I had not the time to make it shorter, and I decided clarity was worth having more text.
I have broken it up into sections to make things clearer.
- I like that it's in sections. I can't confidently predict when I'll be able to respond; I've spent so much time on the Group 3 MS and need to attend RL matters. Sandbh (talk) 08:02, 8 July 2020 (UTC)
- @Sandbh: No problem; please, take your time and give responses when you can. Since it's in sections, if you only have time for one or two at a time, it's also no problem. ^_^ Double sharp (talk) 08:25, 8 July 2020 (UTC)
Fundamental imperatives
If you believe the three building blocks to be "[1] Z ordering; [2] Aufbau principle + Hund’s rule; [3] chemical behaviour" as quoted above, then since Aufbau's principle comes before chemical behaviour, it is clear that Aufbau's principle, which mandates unbroken blocks, should stand unless the chemical behaviour looks so out of place for the elements in question that greater utility comes from breaking it. For helium, this can be granted; for hydrogen, it can be argued. I daresay all the chemists who did their painstaking work in the 19th century separating the rare earths from each other would turn in their graves at the suggestion that lutetium under yttrium was such a case. There is simply not enough power here to overthrow what Aufbau mandates! All of this, e.g. ionic radii, is simply small potatoes compared to the one precedent we really have for this kind of rupturing: beryllium vs neon.
Let me expand a bit for clarity. In the uncontroversial part of our standard periodic table, we see some evidence for what is and what is not important enough to justify a rupture:
- Helium is placed over neon, not over beryllium. The differences between beryllium and neon, chemically and physically speaking, are almost from one side of the chemical universe to the other. So this does not serve us well as a precedent for almost anything, other than maybe floating hydrogen.
- Aluminium is placed over gallium, not over scandium. This despite the fact that in many ways aluminium is more like scandium: they both form +3 ions with the configuration of a noble gas and are in consequence harder than the soft Ga3+ cation. As Rayner-Canham noted, "scandium does more closely resemble aluminum rather than gallium in its chemistry. If hydrogen sulfide is bubbled through a solution of the respective cation, scandium ion gives a precipitate of scandium hydroxide, while aluminum ion gives a precipitate of aluminum hydroxide. By contrast, gallium ion gives a precipitate of gallium(III) sulfide. Also, scandium and aluminum both form carbides, while gallium does not."
So we have one example (Al over Sc) where the chemical resemblances are widely considered not to be enough to justify the rupture of the p block. Now, is the increased similarity of Y to La over Lu anywhere near that even? No, it's even negative, since Lu is in almost every way more like Y than La is. So on this count, (1) La under Y destroys consistency and the relationship to aluminium's position.
I'll also note that lutetium is only barely amphoteric: Lu(OH)3 does dissolve in hot concentrated NaOH, but this only with difficulty. Moreover this may be more about complex formation than about real acidity of the Lu3+ cation, viz. Cu2+, Fe2+, Mg2+ which act the same way forming M(OH)42− complexes. And anyway, Ga is even a little less basic than Al, so there's no problem with Lu being a little less basic than Y. It perfectly follows the inter-block trend: Ga comes after the first d contraction and turns out less basic than Al, so similarly Lu comes after the first f contraction and turns out less basic than Y. (2) This parallel between the d and f contractions is destroyed by the Lu table.
- Building blocks: I would say [1] chemical behaviour; [2] ordering via similarity repetitions; [3] Aufbau etc. I think [3] sorts out what is going on with Sc and Al. I see another conflationary fallacy in your argument: the sky will not fall down. Like H and He, almost every chemist agrees we can leave Al and Sc in their current place in the PTE, bearing in mind their similarities, which Rayner-Canham regards as an (n) (n + 10) relationship, like Ti and Sn; V and P; S and Cr; Cl and Mn; and Xe and Os. Amphoterism: Like Lu, Tl is only barely amphoteric; the oxide only reacts with alkalis when sintered. As noted, there is no fundamental imperative either way for splitting or keeping the d-block together. Parallel destruction: That we do not see a parallel between the first d-contraction and the f-contraction is simply an outcome of the delayed start and finish of the f-contraction. Your argument here is a nice example of the time honoured technique of first setting up a nonexistent straw man and then attacking it. Sandbh (talk) 08:21, 9 July 2020 (UTC)
- @Sandbh: Building blocks. [3] absolutely does not sort out what is going on with Sc and Al. This is already determined by [1] in your scheme: aluminium is more similar to scandium than over gallium, so there is no need to "drill down" further as you have put it: it immediately goes above Sc.
- The fact that almost all chemists agree that Al and Sc are correctly placed is a data point. It shows us that if we want to be claim to be consistent, we cannot move Lu out of the d-block on any grounds weaker than the ones that could be used to argue for Al under Sc, namely stronger chemical similarities that don't yet result in a completely wrong valence prediction (e.g. He over Be). However, your scheme once again results in Lu under Y, as in terms of chemical behaviour lutetium is in the macro scale far closer to yttrium than lanthanum is. Whereas the resemblance Al-Sc is stronger than Al-Ga chemically, the resemblence Y-Lu is stronger than Y-La chemically. So even if you consider Aufbau to be the least important imperative, [1] chemical behaviour already forces the Lu table.
- So this is yet another self-defeating La argument: not only does it fail badly on aluminium, it even shoots itself in the foot and supports Lu under Y.
- What you call the "conflationary fallacy" is generally considered "asking for consistency". If an argument produces nonsense whenever you apply it outside where you would like to apply it, then you have to prove why the situation is so different elsewhere that the argument should not apply. Otherwise it is a refutation, since you are interested in the tapestry of relationships, which by definition must encompass the whole table. That demands a table built on consistent criteria, not on the principle of mal so, mal so: sometimes this way, sometimes that way. If we go that route we shall be able to justify anything at all.
- Parallel destruction. This is a fine example of argument from irrelevancy. Your response to why the parallel should be destroyed is to refer to "the delayed start and finish of the f-contraction". This can only mean one of two phenomena:
- La3+ is [Xe]4f0, lacking an f-electron; whereas Lu3+ is [Xe]4f14.
- La in the gas-phase ground state is [Xe]4f05d16s2, with no f-electron.
- Both are irrelevant. To refute the relevance of (1), we simply note that if counterfactually La were [Xe]4f15d06s2, then no one would ever have started this argument and everyone would agree that La is a member of the f block. Except that this argument would still claim that La must be taken out of the f block because La3+ would still have the noble-gas configuration [Xe]4f0 anyway. That already shows that there is something wrong with it. Not to mention that La3+ is not only f0, it is also d0, and therefore apparently the argument proves it cannot be in the d block either, where you would like to place it.
- To refute the relevance of (2), we simply note that:
- The gas-phase ground-state is chemically about as irrelevant as you can get. It's more important to look at whether the subshell can be occupied and contribute to bonding MOs in compounds. Lo and behold, 4f for lanthanum can enter into service this way, and in so doing actually contribute more to the bonding than 4f in any other lanthanide except cerium.
- Even if we did want to look at the gas-phase ground-states, there is always thorium [Rn]5f06d27s2. Everyone places it into the f-block anyway, including you. You do so on the grounds that in the solid state it has some f occupancy. Well: so does lanthanum. So either you'd have to include La there as well, or you fall back to the principle of mal so, mal so, from which we may justify anything at all. Or you'd have to abandon this argument and look for something else.
- As we can see from this line of inquiry, there is in fact no such thing as a "delayed start and finish of the f-contraction". It begins absolutely on time as lanthanum is the first element with non-hydrogen-like, low-lying 4f orbitals. And it ends on time as ytterbium is the last element with valence-like 4f orbitals whose electrons may be brought out for use in bonding MOs. The same is true for actinium and nobelium using 5f in the next period. The real chemical facts don't give any reason to destroy the parallel at all, and rather confirm what the Lu table is telling us, viz. La through Yb form the 4f row. What the La table tells us is clearly at variance with the facts.
- You may easily refute this argument by showing an example of lutetium actually using its f electrons as valence electrons, or showing that actually all the studies showing f involvement in La are totally wrong. (The last one not terribly likely, given the need to invoke them to explain cubical complexes on symmetry grounds.) I notice that there still appears to be no avenue given to refute your arguments. Or even what apparently seems to be an axiom for it, i.e. the "delayed start and finish of the f-contraction" that for the nth time I have just demonstrated does not exist in any chemically meaningful sense.
- If I continue to not receive any sign that your approach is falsifiable, then I plan to start the RFC for switching to the Lu form as previously stated. Double sharp (talk) 08:42, 9 July 2020 (UTC)
Where each subshell starts
The more important thing is not whether the subshell is occupied in the gas-phase ground state, which is about as far from chemistry as you can possibly get. It is whether the subshell is participating in chemical bonding. As you know and apply to thorium. By this chemically relevant standard, lanthanum and actinium have started filling the f subshells. No consistent standard allows you to cut off La and Ac from the f block without also cutting off Th. Which I see you clearly don't want to do. So, here too, (3) La under Y destroys consistency and a relationship to thorium in terms of understanding the meaning of electron configurations. (And also palladium, which uses its 5s shell despite it being empty in the gas-phase ground-state.)
- This is an example of drill-down obfuscation. Gas-phase ground states are widely referred to in chemistry, as you know, and for a first-order model, highly successful. Th, as we have discussed, is an example that effectively nobody loses any sleep over, and which we leave where it is (under Ce) noting its interesting free atom and condensed phase configurations. Ditto Pd. I am relying here on the approach by Schwerdtfeger, Smits & Pyykkö (2020) that, "almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive…nature in mind." I also agree with them when they say: "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE." Th is another one of those unnecessary disputes. Sandbh (talk) 03:51, 10 July 2020 (UTC)
- @Sandbh: That's mal so, mal so as its finest. Apparently for you the La vs Lu controversy is an isolated thing and we cannot even note that arguments for La would lead to questioning standard placements because those standard placements are standard and cannot be questioned. Which is a fine example of not understanding reductio ad absurdum coupled with self-contradiction, as such an argument collides head-on with the desire to look at the rich tapestry of chemical relationships. Which by definition extend across all elements. Of course, it goea both ways: if no one loses sleep about Th under Ce, then why should they lose any sleep about Lu under Y?
- Also, this is the first time I've ever heard of a first-order model that has such funny irregularities as gas-phase configuration does. Naturally, the idealised fuzzy configurations I advocate and that are apparently close to what Droog Andrey actually teaches have no such thing, they're absolutely linear following the Aufbau principle. That's what a first-order approximation is like. You can't get much simpler than linearity as a model.
- See you at the RFC (to be drafted by me and started in the next few days). Double sharp (talk) 04:25, 10 July 2020 (UTC)
- @Double sharp: I feel you will be wasting your time before (a) my article appears on-line; and (b) the report of the IUPAC Group 3 project group appears. But suit yourself. Sandbh (talk) 08:13, 10 July 2020 (UTC)
- @Sandbh: We'll see how the consensus develops there before we decide on whether or not I'm wasting my time. Let's just note that the split in opinions here seems to be:
- Supporting the change to Lu: me, DA, Dreigorich, CR, Officer781
- Supporting the retention of La: you, for now apparently R8R, but let's see what he says in his words: "I would be opposed to a change in English Wikipedia right now, since Wikipedia is a tertary source, and it needs a good precedent for a change that could not qualify as original synthesis (or, as the local terminology goes, original research). I do think that a decision from IUPAC could be authoritative enough to overrule that if editors agreed on that; importantly, because the decision was made by somebody else, someone we could consider authoritative. I will also note that the current version was fixed in a RfC, which has a wider scope than a vote in our relevant but small project, and we'd need an RfC to override that decision. There are house rules here in English Wikipedia, and we have to respect them. At the same time, if I were writing my own self-authored book and were not subject to any house rules, I'd be very fine using a -Lu-Lr table." That doesn't seem to indicate that he's terribly convinced by your arguments even if he's opposing the change at the moment.
- So, if this were considered a binding discussion by itself, as happened for our decision to recolour our periodic tables, then it seems to me that on the Lu side we have managed to get an airtight 2/3 majority. A 5/7 one in fact. So, I don't think the RfC is going to be a waste of time at all. ^_^ Double sharp (talk) 11:15, 10 July 2020 (UTC)
- @Sandbh: We'll see how the consensus develops there before we decide on whether or not I'm wasting my time. Let's just note that the split in opinions here seems to be:
- @Double sharp: I feel you will be wasting your time before (a) my article appears on-line; and (b) the report of the IUPAC Group 3 project group appears. But suit yourself. Sandbh (talk) 08:13, 10 July 2020 (UTC)
@Double sharp: The issue is not support within our project. The issue is, per R8R, the predominance of the La form in the literature, as noted by the IUPAC Group 3 project team, and the IUPAC project itself. Sandbh (talk) 02:32, 11 July 2020 (UTC)
- Not much of a predominance, that. There are many periodic tables besides the ones that appear in textbooks. Indeed probably most people's first periodic table was not in a textbook. Just see how much La dominates over * if you do that.
- As for following the textbook literature: ah yes, full of textbook errors, isn't it. Somehow we're allowed to refer to research papers to refute the d-orbital explanation of hypervalence even though it probably goes well against the textbook battleship. For refuting La under Y that's verboten. Double sharp (talk) 05:19, 11 July 2020 (UTC)
One specimen of the repetitive debate in a nutshell.
- A: La must go under Y, as the first 4f electron in the gas-phase appears only in Ce!
- B: By that logic Th must go under Hf, as the first 5f electron in the gas-phase appears only in Pa.
- A: No one loses sleep about that.
- B: By that logic no one should lose sleep about Lu under Y either. And in fact, the fact that your La argument questions something that no one loses sleep over proves that there's something wrong with it.
- A: You extend my argument beyond its context. I have no desire to take on the chemical establishment.
- B: Then why are you using an argument that does?
- A: Because that is not my argument. I only apply it to La and not Th.
- B: So why is Th so different from La that we cannot apply it the same way? You cannot artificially restrict context, or else we may prove anything.
- A: In the condensed phase Th has some f occupancy!
- B: But so does La!
- A: Any 4f occupancy in La is minor, only a tipping point argument, and not important.
- B: Then why is an exactly similar level of 5f occupancy suddenly important for Th?
- A: La must go under Y, as the first 4f electron in the gas-phase appears only in Ce!
Lather, rinse, repeat. Double sharp (talk) 04:39, 10 July 2020 (UTC)
- @Double sharp: That's an invalid specimen since the debate hinges upon on many other considerations. Sandbh (talk) 02:32, 11 July 2020 (UTC)
- And it's a good example of drill-down obfuscation. Sandbh (talk) 02:33, 11 July 2020 (UTC)
Continuums and breaks
What is so different between group 12 and group 11? The d electrons are already getting weakly involved, just look at increased stability of Cu(II) and even Ag(I). You likewise see low coordination numbers. It's simply a matter of the peripheral groups of a block always being somewhat transitional, because everything is a continuum here.
Indeed, not understanding this is part of what drives arguments like "let's rip group 3 away from the more transition-like groups" to justify Sc-Y-La. Never mind that, by this logic, Al also has to be ripped away from the p-block to go over Sc: it's more pre-transition-like than post-transition-like. And never mind what this does to Ti, Zr, Hf, and Rf with their +4 cations that continue a trend: +1 cations require less acidity to exist than +2 cations than +3 cations than +4 cations, but they all exist in water at the right pH. And you can see that they're real cations because the precipitate from the hydrolysis will redissolve in hot concentrated HCl (at least if it hasn't aged too much). Contrast that to elements which really don't have aqueous cations because such would immediately and irreversibly attack water like boron. For once there is a real break here because we are focusing only on water. Excluding group 4 pushes it inwards from this natural break completely artificially.
So, here (4) La under Y is based on a false break, inconsistently ignores aluminium, and ignores the real break that would demand a d-block rupture between groups 4 and 5 instead. Not to mention between the 3d row and the heavier d rows, based on the difference in stability of aqueous cations.
- Difference between group 11 and 12: There is an abrupt and significant reduction in physical metallic character from group 11 to group 12. Their chemistry is that of main group elements. Al is yet another example of an element the placement of which nearly every chemist would agree is fine where it is, noting its distinctive nature. Here I am drawing a distinction between the one element one position proposition which ought to apply to the La or Lu question, in contrast to all other element placement questions that effectively all chemists agree can stay where they are. The latter are examples of obfuscation by irrelevant extension arguments. Sandbh (talk) 04:02, 10 July 2020 (UTC)
- Widely referred to by all practicing mathematicians as reductio ad absurdum. Double sharp (talk) 04:46, 10 July 2020 (UTC)
@Double sharp: If I understand you right, then see if the chemists could care, or that the chemists would enjoy being told by the mathematicians what to do. Sandbh (talk) 05:14, 10 July 2020 (UTC)
- @Sandbh: It's also a principle of formal logic. If you would like to claim that chemistry, a science, is not based on logic, then see if any chemist will not be offended. Double sharp (talk) 05:38, 10 July 2020 (UTC)
The proof of the irrationality of the square root of 2, as argued between a member of Pythagoras' school and someone subscribing to Sandbh's school of logic. Classics scholars, I apologise deeply for all the anachronisms.
- B: I have made a most marvellous discovery. The square root of 2 is irrational.
- A: The mathematics battleship begs to differ. Such issues have never made anyone lose any sleep.
- B: This is what our great teacher Pythagoras has demonstrated. Two can play the game of credentials.
- A: It is self-evidently obvious that we can get anywhere on a line simply by cutting it into fractions. Between any two rationals there will always be another, its average.
- B: If the square root of 2 was rational, then it may be represented as a fraction. Do you agree?
- A: Yes, that is the definition.
- B: So let us call it a/b, a and b integers, the fraction in lowest terms. So a and b cannot both be even.
- A: Of course.
- B: Then we agree that a2 = 2b2 by simple rearrangement, whence a being even.
- A: Certainly.
- B: In which case a = 2c for some integer c. But then 2b2 = 4c2. Cancelling a factor of two on both sides we get that b is also even. Which we just said could not happen.
- A: Your argument is a one-shot based only on oddness vs evenness. The properties of even and odd numbers are something else, I have no desire to upturn the mathematical establishment when it comes to these uncontroversial properties. We have analysed a lot of philosophical reasons for why the rationals should be all the numbers!
- B: How is this a one-shot? It relates the question all the way back to fundamental properties of integers! And I refuted the argument!
- A: But that is not my argument! I only say that it's a fraction with the numerator even!
- B: Then why am I not allowed to make the inference about the denominator being even too?!
Double sharp (talk) 05:14, 10 July 2020 (UTC)
Lanthanides as a silly category
So, just count how many authors talk about the lanthanides without including lanthanum. Even those who define the Ln to exclude La usually put it in "for comparison", which is a good sign that a Ce-Lu category is unnatural. And just look at how many also include yttrium "for comparison" because its properties are so similar to those of the late lanthanides (especially Dy). I stand by my words: it's clearly a silly category if taken literally because pretty much everybody supplements it with yttrium. The category people are really using not only includes La in the lanthanides, but also seems to include yttrium as an honorary lanthanide.
The idea that lanthanum is not a lanthanide, and that actinium is not an actinide, is chemically and linguistically extremely strange. Chemically, because we all know that La behaves perfectly normally for an early lanthanide, and it even always occurs with them in nature. Linguistically, because the whole point of the "lanthanide" category is to collect together elements that behave like lanthanum. Clearly, there is no element that behaves more like lanthanum than lanthanum itself. Booting it out of its own category is simply obtuse pedantry that fails to help understanding of anything at all. Not only that, but placing lanthanum there makes for a hilariously awkward transition metal: by every measure, lanthanum is an outlier among the early d elements, but a perfectly normal early f element. Meanwhile, booting lutetium out of the lanthanides makes a lot more sense because it is, indeed, the most extreme lanthanide in the direction away from lanthanum. (Because it's the smallest one.) And it, by every measure, acts quite reasonably for an early d element, and is a far better bedfellow of hafnium, tantalum, tungsten, and rhenium than lanthanum can ever hope to be.
So, here we see that (5) La under Y gives the mistaken impression that more difference exists between La and the other lanthanides than there really is, and unnecessarily breaks apart chemically similar elements from each other.
- No less than the highly respected G&E take exactly the approach you disparage. They treat Sc-Ac in one chapter and the Ln (Ce-Lu) in another. As does Wiberg: one chapter for Sc-Ac; another for Ce-Lu. And C&W, too, treat the Ln as Ce-Lu. How's that for a big 3 trifecta? Do any of them worry about La as an awkward TM? No. Do any of them feel the need to boot Lu out of the Ln? No. Why should anybody else feel they need to waste time on these sleep-sapping issues? Sandbh (talk) 04:35, 10 July 2020 (UTC)
- @Sandbh: So, just see how many times Y and La are mentioned in the section supposedly about the Ln. See also Cotton's own approach in Lanthanide and Actinide Chemistry.
- Re characterisation of these issues as "sleep-sapping". Are we trying to find what the best composition of group 3 is, or not? Double sharp (talk) 04:45, 10 July 2020 (UTC)
- Yes, some fuzziness is fine, and worth noting. That's not the same Cotton. Yes the group 3 issue is worth discussing per Scerri's maxim of one element one place. The rest is not worth losing any sleep over, as opposed to noting, including in different contexts, such as earth science, metallurgy, philosophy, symmetry etc. So I'm saying La is the go, and we should retain our awareness of all the other wrinkles. Sandbh (talk) 05:09, 10 July 2020 (UTC)
- @Sandbh: You're right: it's not the same Cotton, my bad. Still, the other one is also a significant figure.
- I have demonstrated that there are no important wrinkles here. Lu is the go according to everything important. Including all of what you mention. Double sharp (talk) 05:17, 10 July 2020 (UTC)
Lu and Lr as terrible f block elements
There are two simple reasons why they have not yet been firmly booted out of there by everybody. Although, as we know, there are many fervent advocates of so doing, including most people who actually analyse the subject.
- The true badness of Lu and Lr as f block elements isn't obvious until you actually understand the configurations and molecular orbitals involved and distinguish periodic table placement from chemical similarity. The case of aluminium not going over scandium should make that obvious already. Because one important way in which they are absolutely terrible f elements is that they never use their f orbitals as bonding MOs. You clearly can't understand that without MO theory and calculations. (I once again remark on how long it took to disprove d involvement in hypervalent molecules like PCl5.)
- Inertia. In terms of chemistry, lutetium in the f block does not look obviously terrible. (It is a bit weird after the slight stabilisation of +2 you see for Tm and Yb, that's all.) That's what I said above about the differences being small potatoes cutting both ways: most chemists have not felt the need to make the change because the differences Y-La vs Y-Lu are pretty small. But note that the same was true for Mg-Zn and Al-Sc, now both distant memories only.
Lawrencium in the f block indeed looks terrible: it's not a good late actinide at all. A late actinide (Es through No) typically forms a divalent metal, is not so dense, is probably not so hard, is probably not quite so electropositive, and has a significant +2 oxidation state (the later the more significant). Lawrencium stuck to the end of this trend breaks everything. That's nothing like how scandium appears at the beginning of the d-block, it already has incipient transition metal properties in how it can tolerate the +2 oxidation state in compounds like CsScCl3. Or how zinc appears at the end of the d-block as the reasonable closure to a trend of increasing stability of the +2 over the +3 state going from Fe to Zn (quite a bit like the position of nobelium at the end of the f-block). No, it looks exactly like how it would look if you stuffed gallium as the "culmination" of the d-block trend. After all that work the trend does to stabilise +2 over +3, suddenly Ga goes out in favour of +3 and declares that it's totally done with the +2 state. I daresay everyone would find this strange. But it is exactly what Lr looks like after that exact same thing goes on from californium to nobelium.
But, since to a first approximation about 0% of chemists actually care about the chemistry of the mayfly lawrencium for their work, with its half-life of about eleven hours, the terrible fit is tolerated, because it mostly goes unnoticed in the average chemist's professional life.
So, we can see that (6) La under Y going unreproached has a lot to do with the clearest counterexample of lawrencium simply not being long-enough lived for the average chemist to care. That is bringing in nuclear processes into the periodic table and declaring the short-lived elements to be second-class citizens just because they don't live very long. You don't stand for this for astatine, universally treated in schoolbooks giving periodicity exercises as if it were going to be the perfectly normal fifth halogen, despite it being much more like a metal. Why turn a blind eye to this same injustice for lawrencium?
- I think this is another good example of drill-down obfuscation. We already know Lr is predicted to behave as a normal actinide. We know the most stable oxidation state for the later An, from Am-95 to Lr-103 is +3, with the only exception being No with +2. and even here it will still form +3. And that's all that needs to be said, as a first order phenomenon. The rest of it is nice to bear in mind but nothing worth losing any sleep over. As for Lu, we know it shows the culmination of the f-electron-induced lanthanoid contraction, which starts at Ce and reaches its maximum at Lu, before subsiding in Hf and its successors, due to better shielding by the extra 5d electrons. Sandbh (talk) 04:57, 10 July 2020 (UTC)
- Yet more reassertion of things that have long since been refuted n+1 times over. And of course, with an analysis that is totally based on a "Main screen turn on!" interpretation of oxidation states, forgetting that even for a first-order rationalisation redox behaviour must be included for it to have any meaning. And at variance with what I've already quoted from Jørgensen. He must be wrong, of course. Funny how sources are only wrong when they support Lu. Double sharp (talk) 05:40, 10 July 2020 (UTC)
Double periodicity
As I said, this isn't about matching tranches. It's about making sure the elements at the end of each tranche behave like a half-filled or full-filled subshell element ought to. Such a subshell should be harder to delocalise. Therefore you should see lower melting and boiling points (weaker metallic bonding), a more stable +2 state (not going beyond the outer s electrons), that kind of thing. It's not about just showing the same trend between the tranches. Otherwise it's impossible to tell which is better because obviously Eu-Gd parallels Yb-Lu. But one can only justify that train of thought if one doesn't stop and think and ask: why on Earth should one want such a trend for the end of a block? What should the end of the block mean if the electron configuration means anything for chemistry? What does it mean when we see it in the 3d row?
Eu and Yb take these positions for every chemically or physically relevant trend. Never Gd and Lu. And Eu and Yb in the f block always look more or less like Mn and Zn do for their positions in the d block. Just look at 3rd ionisation energies (the relevant one, getting rid of the s2 electrons so that we take electrons out of the characteristic d or f orbital), electronegativity (dips for Eu and Am just like it does for Mn and Tc; the Pauling value for Eu is not quite so general and should be lowered to reflect its common and stable +2 oxidation state in which it's more electropositive, but in any case Am shows the trend perfectly), melting points, boiling points, densities.
So, we can see that (7) La under Y promotes formal similarity over the true meaning and causation behind the effects we see; and it destroys an otherwise compelling parallel between the d and f transition blocks.
- I see this is another example of drill down obfuscation, beyond what is needed for a first-order arrangement. It doesn't matter what you think double periodicity is about. Not that it isn't worth bearing in mind. I look to the literature, and Klemm (1929), Remy (1956), Ternström (1976), Shchukarev (1974), Sobolev (2000), and Rokhlin (2003). You may however consider all of them to be wrong. Sandbh (talk) 05:23, 10 July 2020 (UTC)
- So just look at high-school chemistry textbooks and how they explain the first IE dip going from N to O, or the low mp of Mn and Zn compared to the rest of the 3d row. It doesn't matter what you call it. I am focusing on the cause, you only on superficial matches. By such logic we may start every period in group 2 and end it at group 1. The trends all match, periodicity is still there, the noble gases are simply imitators of the alkali metals closing their shell early. I trust this reductio ad absurdum will convince everyone here but Sandbh. Double sharp (talk) 05:49, 10 July 2020 (UTC)
Cations
The argument about +3 cations doesn't hold any water. Suppose, counterfactually, that lanthanum really were [Xe]4f16s2 in the ground state. Then I doubt you would be arguing for the f block to start at Ce. Actually I doubt anyone would be arguing that. But alas! That f electron would still go missing in the +3 cation, and the argument would still support Ce-Lu!
And not to mention the silliness that results when applying that argument to the s block. Look, when I take the +1 cation of all the group 1 elements, they don't have any s electrons at all. So clearly they must go before the s-block. Actually, the same thing happens when I take the +2 cation of all the group 2 elements. And no one can fault me for picking those oxidation states, they're clearly the characteristic ones for those elements. So, clearly there are no s-block elements at all, as they are all s0 and must go before the s-block just like La3+ f0 must go before the f-block. Oh, except La3+ is also d0 and therefore we can't put it in the d-block either like this argument is trying to justify. Actually it's also p0 and s0, so we have to float it out of the periodic table entirely. It's a self-defeating argument that obviously results in nonsense whenever you apply it outside the f-block.
So, we can see that (8) La under Y is partly based on one-shot and totally local arguments that ignore global considerations. And there is no "rich tapestry of chemical relationships" without global considerations.
- @Double sharp: This is another example of obfuscation by irrelevant extension. (1) to an out-of-universe hypothetical situation; (2) to other blocks in which there are no delayed starts to filling the applicable sub-shells. Sandbh (talk) 05:36, 10 July 2020 (UTC)
- Yet more refusal to accept the principle of reductio ad absurdum, universally accepted by anyone who wants his or her science to be based on standard, classical, logic. Double sharp (talk) 05:42, 10 July 2020 (UTC)
- @Double sharp: Go tell it to the chemists. Sandbh (talk) 07:46, 10 July 2020 (UTC)
- @Sandbh: Sure! Go and tell them that logic is irrelevant to their discipline. See how much they like it. See how much they agree. Double sharp (talk) 08:21, 10 July 2020 (UTC)
Number of fds electrons
You are not comparing like with like here. You are getting a count of 17 fds electrons for Lu and Lr by counting the fourteen f electrons they have too. Despite the fact that those are core electrons, never used in bonding MOs. Well: by such logic we may likewise count the 4f electrons for hafnium through radon, too, which have about as much to do with the bonding as the 4f electrons in lutetium; they just provide incomplete shielding effects for all of Lu-Rn. And the same for the 5f electrons for rutherfordium through oganesson, too, which have about as much to do with the bonding as the 5f electrons in lawrencium; they just provide incomplete shielding effects for all of Lr-Og. So it seems that Hf through Rn are letting the team down as much as Lu is. Better have a periodic table that just sprawls out to the right like this, then:
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og
That's the only consistent form of this approach. If one claims Lu doesn't fit under Y, because Lu has 17 electrons in those shells whereas Y has 3, then clearly Ga with 13 electrons in dsp can't go under Al with 3 either.
The chemical worth of this form is left as an exercise to the reader. Especially when it comes to the amazing resemblances it asks for like xenon to hafnium.
So, we can see that (9) La under Y promotes the false idea that the f electrons in Lu and Lr can ever be used as valence electrons, and that their f orbitals may be used as bonding MOs. No such thing happens.
And (10) La under Y obscures that the true rôle of the f electrons in Lu and Lr is simply as weakly shielding core electrons providing incomplete shielding effects, therefore obscuring the true similarity of them to Hf-Rn and Rf-Og, and destroying a parallel to the elements Ga-Kr after the first d block row.
- I think this is an example of a "conflationary fallacy". By this I mean taking an argument of a fixed scope, conflating it with a larger scope, and then arguing that since the conflated argument does not hold, neither therefore does the smaller scope argument. There may be a more proper term for this phenomenon; if so I don't what it is. An example would be arguing that since quantum phenomena don't hold at a macro scale, they therefore have no validity.
- My limited scope argument was to observe that if the f-block is La-Lu or Ce-Lu, where La is lined up with group 3, and Ce is lined up with group 4, then the fds count for each f-block member matches the group numbers they are aligned with. Thus:
[A] f-block as La-Lu [B] f-block as Ce-Lu 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 ** Hf Ta W Re OS Ir Pt Au Ag Tl Pb Bi Po At La Hf Ta W Re Os Ir Pt Au Ag Tl Pb Bi Po At -------------------------------------------- -------------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
This does not work so well with the f-block as La-Yb:
[C] f-block as La-Yb 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Lu Hf Ta W Re Os Ir Pt Au Ag Tl Pb Bi Po At -------------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
- I say, "not so well" as, in options [A] and [B] the fds count is consistently applied across La and the lanthanoids whereas in [C] it cannot be applied to Lu in group 3, as Lu as 17 fds electrons.
- The conflationary fallacy is to extend the scope of my argument to:
- Hf to Rn;
- core electrons, never used in bonding MOs;
- Lr-Og;
- the entire periodic table;
- Ga with 13 electrons in dsp therefore can't go under Al with 3 either;
- the amazing resemblances it asks for like xenon to hafnium;
- La under Y promotes the false idea that the f electrons in Lu and Lr can ever be used as valence electrons, and that their f orbitals may be used as bonding MOs;
- La under Y obscures that the true rôle of the f electrons in Lu and Lr is simply as weakly shielding core electrons providing incomplete shielding effects, therefore obscuring the true similarity of them to Hf-Rn and Rf-Og, and destroying a parallel to the elements Ga-Kr after the first d block row; and
- at the same time throwing in an apples and oranges argument.
Setting aside the above fallacy, what is left standing is my original argument. Sandbh (talk) 06:18, 9 July 2020 (UTC)
Four fallacies
- @Sandbh: The above in fact provides examples of logical fallacies, but they are on your part, not mine. I'll go over them now.
- Since you are concerned with the "rich tapestry of chemical relationships", we must look at how well the argument works as a general principle. An argument that can't be applied to the whole periodic table is immediately weakened. If it cannot apply to the d-block, then why should we take what it has to say for the f-block seriously?
- We went over this in Archive 42. Let me quote it for you.
| “ | @Double sharp: If you said, "the f-block must end when the final f electron appears, i.e. at Yb" I'd ask, "is this statement impacted by aufbau irregularities(?)"; and, "is it impacted by where the f-block then starts when the first f electron appears?" I wouldn't argue about the start of the second f-block row, since this not part of your argument. And I wouldn't argue about the d-block because that's not within the scope of your argument. Sandbh (talk) 22:59, 20 February 2020 (UTC)
@Sandbh: You should be arguing about the d-block if I said that. Because the natural scientific response to me going "oh, this is only valid for the f-block" is not "Great Scott! You're right, of course. Let me fight within the f-block only". It is "so what is the reason the f-block is categorically different from the other blocks that gives you a right to say so"? See the next few paragraphs. ;) [Which I don't quote again now, because this is the important point.] And you should also argue what this criterion has to do with chemistry and physics, since it is not unheard of elsewhere that an already preemptively filled subshell is still valence later (see group 12 in the d-block), and it would be my burden to prove this doesn't happen for Lu. (Which Droog Andrey and I have done.) Double sharp (talk) 23:07, 20 February 2020 (UTC) |
” |
- In other words: before you claim that it's a fallacy to extend the argument to Hf onwards, you need to show why the situation for Hf onwards is categorically different. Otherwise, excluding those cases which torpedo the argument is completely arbitrary. And that would be a fallacy because by such logic one can equally well "prove" anything by simply ignoring all counterexamples by saying "my argument doesn't extend to them".
- There is a name for a similar logical fallacy, indeed. It is called No true Scotsman. The only difference here is that here we arbitrarily limit the scope of the argument rather than the definition. So we count: (11) Some La arguments are arbitrarily limited in scope in order to avoid the absurdities they produce when applied elsewhere.
@Double sharp: You don't understand how chemistry works. Go back and read Schwerdtfeger, Smits & Pyykkö (2020) and their reference to leaving H and He where they are, bearing in mind their distinctive nature. The other thing you don't understand is the nature and value of first-order approximations. It's not always necessary to drill down. You can if you're so inclined but it's not necessary to appreciate the broad contour of the situation. Sandbh (talk) 07:55, 10 July 2020 (UTC)
- @Sandbh: I'll be happy to not "understand how chemistry works" since it puts me in the same boat as Eric Scerri writing in p. 173 of his Collected Papers In the Philosophy of Chemistry (my bold):
| “ | The fact that such a variety of successful [periodic] systems can coexist, more or less peacefully, suggests that how the periodic system is represented is not a crucial issue. No particular representation is refutable, as a theory might be, unless it commits internal inconsistencies. | ” |
- And while I disagree about refutability, with the logic that a scientific statement kind of by definition has to be able to be tested against the scientific method, note those revealing words I bold. La over Y keeps on tripping on this.
- It's also quite funny to accuse a Lu proponent of not understanding the nature and value of first-order approximations. After all, the Lu table basically takes the Madelung rule as its even simpler first-order approximation than ground-state gas-phase electron configurations. XD Double sharp (talk) 11:02, 10 July 2020 (UTC)
@Double sharp: There are a couple of things to say about Scerri. 1. Whenever you quote him, especially his earlier work, you need to check his later work to see that he has not changed his position, which he is known to do 2. I've occasionally seen him say one thing only for him to seemingly contradict himself elsewhere. In the same book, in the introduction, Scerri writes, "The failure to reduce chemistry to electronic configurations is hardly surprising since configurations represent an approximation that is strictly inconsistent with quantum mechanics." (p. 16?). This is the approximation that form the basis for understanding chemistry. And yet it is supposed to be inconsistent with quantum mechanics? Eh?
You do understand the nature of first order approximations. We've talked about this concept previously. The issue is that to refute La you are obliged to engage in drill down obfuscation, or obfuscation by irrelevant extension, or conflationary fallacies—what Schwerdtfeger, Smits & Pyykkö (2020) had in mind when they said, "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE." Meanwhile the pachyderm in the room i.e. the first order approximation, remains unscathed and continues to be used the world over by chemists.
The Madelung Rule is a "too simple" approximation—"too simple" being a conceptual criticism I picked up from you. Mind you it is nevertheless a very good way of demonstrating the notional symmetry underlying the periodic table, pre-symmetry breaking/turbulence, and the resulting 20 anomalies. Sandbh (talk) 04:10, 11 July 2020 (UTC)
- Now, there happens to be another fallacy going on here, inherent in your treatment of the f electrons of lutetium and lawrencium. This is the fallacy of (12) prioritising form over function, which is another common failure of the La table. You look at the number of electrons, but you don't ask if they are used the same way. For La through Yb, the fds electrons are all valence electrons. Indeed, every type of orbital can be used. But for Lu that's not true, only the d and s electrons are. That's the same as with Hf through Hg.
- So now one asks: what is the sense of counting non-valence electrons in a periodic table that is supposedly based on chemistry? They don't participate in chemistry. All they do is provide incomplete shielding effects. And we surely can't reflect them, because we agree that Al belongs over Ga, even though Ga has an incomplete shielding effect from 3d electrons that Al simply doesn't have. So we can see another failing of the La table: (13) its arguments sometimes fail to be based on chemically relevant data. That's true of the "but in the gas phase La has no 4f argument" too, incidentally.
- If we edit the argument to focus on the number of valence electrons, then it strongly supports the f-block as La-Yb. Let's match group numbers.
[C] f-block as La-Yb 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po ----------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
- Whereas Lu lets the other two tables down thanks to its f electrons being core electrons:
[A] f-block as La-Lu [B] f-block as Ce-Lu 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 ** Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At La Hf Ta W Re Os Ir Pt Au Ag Tl Pb Bi Po At -------------------------------------------- -------------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 3 4 5 6 7 8 9 10 11 12 13 14 15 16 3 4 5 6 7 8 9 10 11 12 13 14 15 16 3
- So, when corrected to focus on relevancies, the argument ends up strongly supporting Lu in group 3; whereas as you have it the argument can't tell between the La table and the * table. (14) La arguments, when corrected to be based on chemically relevant data, often end up supporting the Lu table, sometimes even more strongly than their uncorrected versions supported La.
- This is another example of drill down obfuscation. It is not necessary to consider valence electrons in order to observe the regularity in question. Sandbh (talk) 08:10, 10 July 2020 (UTC)
- @Sandbh: But they are for your 234 maximum oxidation states argument. Since oxidation states are formed because of chemical activity of the valence electrons. Mal so, mal so again. When something seems to support La it is a pleasing observation, when it supports Lu it is drill-down obfuscation. I see Droog Andrey was right after all. XD
- This is another example of drill down obfuscation. It is not necessary to consider valence electrons in order to observe the regularity in question. Sandbh (talk) 08:10, 10 July 2020 (UTC)
| “ | And we all know that reason: your devotion to La table :) Droog Andrey (talk) 11:51, 23 January 2020 (UTC) | ” |
- At least I use logic to attack the La table. Which is, by pretty much the entire scientific community, including the chemists, considered something usually relevant to scientific discovery and analysis. Let me quote you from Archive 42, then:
| “ | And you are the voice in the wilderness saying to the entire chemistry and classification science communities that they are all wrong. Good luck with that. Sandbh (talk) 05:27, 15 February 2020 (UTC) | ” |
@Double sharp: "But they are for your 234 maximum oxidation states argument." That's right. It's not necessary to consider valence electrons in order to observe the regularity in question. OTOH, for the 234 triads, it is, an argument which you seek to refute via obfuscation by irrelevant extension e.g. since QM only applies at the quantum scale and not at the macro scale, it follows that QM don't exist. Sandbh (talk) 04:17, 11 July 2020 (UTC)
- As we can see, there seems to be no criterion for what is necessary to observe other than Sandbh's own decisions.
- The interested reader may note that there are such things as Macroscopic quantum phenomena. Including the superconductivity that provides so many nice arguments for why Lu should be in group 3. The interested reader who considers logic to be the key to scientific endeavour, unlike Sandbh who is in the wilderness on this one, may also be amused by the incongruity between the scope of applicability of QM, in which some reason is given why typically quantum phenomena don't appear at the macro scale, versus those of his arguments, for which no reason is ever given but his own arbitrary decisions. Double sharp (talk) 04:28, 11 July 2020 (UTC)
- There is even a third mistake here. That mistake is referring to Lu as something that lets the team down in the Lu table. Let me quote you:
| “ | My limited scope argument was to observe that if the f-block is La-Lu or Ce-Lu, where La is lined up with group 3, and Ce is lined up with group 4, then the fds count for each f-block member matches the group numbers they are aligned with. Thus:
[A] f-block as La-Lu [B] f-block as Ce-Lu 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 ** Hf Ta W Re OS Ir Pt Au Ag Tl Pb Bi Po At La Hf Ta W Re Os Ir Pt Au Ag Tl Pb Bi Po At -------------------------------------------- -------------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu This does not work so well with the f-block as La-Yb: [C] f-block as La-Yb 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Lu Hf Ta W Re Os Ir Pt Au Ag Tl Pb Bi Po At -------------------------------------------- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb I say, "not so well" as, in options [A] and [B] the fds count is consistently applied across La and the lanthanoids whereas in [C] it cannot be applied to Lu in group 3, as Lu as 17 fds electrons. |
” |
- As we can see, the argument you bring out is about "the fds count for each f-block member". Then you claim that Lu does not work so well in a Lu table, but fail to appreciate the most important feature of a Lu table: it makes the claim that lutetium is not a member of the f-block. Therefore this invokes the fallacy of false equivalence. The f-block sets argued for by the tables are not the same. If you have limited your scope to the fds counts of the f-block members, then Lu cannot be a counterexample for the Lu table because that table claims that Lu is not an f-block member. The only way in which Lu could possibly be considered a counterexample is if you have already decided in advance that Lu is an f-block member, which is tantamount to saying that the La or * table is right from the beginning, in which case obviously any comparison is unfair. So (15) some La arguments only work by implicitly using the fallacy of begging the question.
- Of course, in that case you cannot decide between the tables. They all work perfectly well, as expected since they choose their definition of what's in the f-block. Which is why I refer to whether the orbitals in question are valence orbitals, as an independent means of verifying "f-blockness": do they at least use 4f as a bonding MO?
- Those are the fallacies I see in your argument, respectfully. Double sharp (talk) 08:14, 9 July 2020 (UTC)
- There is no fallacy here. I start with the f-block or Ln as seen in the table used within IUPAC, which I regard as being down towards the chemistry end of Scerri's near continuum of PTs. I count the fds electrons and note there is a good alignment with groups 3 to 17. Or rather, there used to be. Whoever designed the latest iteration of the the "IUPAC" table has misaligned the 15-column f-block in order to make room for the stupid logo! Anyway, that aside, when we move from the 15-column form to the 14-column version we see this works for the Ce-Lu version but not so well for the La-Yb version. There is no need to drill-down and look for a convoluted logic-based argument in order to refute my approach, and argument which, in this case, has no relevance. A conflation fallacy in other words. Thank you, Double sharp. Sandbh (talk) 08:10, 10 July 2020 (UTC)
- Incredible. You start with the f-block as seen in the * table, in which Lu is claimed to be an f-block element. So of course the Lu table will never look good because you've assumed a different form to start with. Begging the question. With every reply from you the fallacies become more obvious. But of course, logic is claimed to be "convoluted" and "drill[ing] down" whenever it refutes your points. Double sharp (talk) 08:19, 10 July 2020 (UTC)
- There is no fallacy here. I start with the f-block or Ln as seen in the table used within IUPAC, which I regard as being down towards the chemistry end of Scerri's near continuum of PTs. I count the fds electrons and note there is a good alignment with groups 3 to 17. Or rather, there used to be. Whoever designed the latest iteration of the the "IUPAC" table has misaligned the 15-column f-block in order to make room for the stupid logo! Anyway, that aside, when we move from the 15-column form to the 14-column version we see this works for the Ce-Lu version but not so well for the La-Yb version. There is no need to drill-down and look for a convoluted logic-based argument in order to refute my approach, and argument which, in this case, has no relevance. A conflation fallacy in other words. Thank you, Double sharp. Sandbh (talk) 08:10, 10 July 2020 (UTC)
Keeping in mind the distinctive quantum nature of H and He
This is a revealing quote. Evidently, sometimes the increased chemical similarities are better to show, while keeping the distinct quantum natures in mind (H and He). And sometimes the quantum similarities are better to show, while keeping the chemical relationships in mind (Be and Mg). Why does one have priority in one case and the other have it in the other case? What are the rules? Without such rules, this cannot be considered a consistent periodic table. Rather, it becomes a periodic table of mal so, mal so: sometimes this way, sometimes another way. Is that what we want to crown as the foundation of chemistry?
One element, one place, indeed! So in what sense are the similarities of Y to La so overwhelming as to overthrow the Aufbau-mandated placement of Lu? In no sense at all, as I said, because actually chemical and physical properties both agree that lutetium is far more like yttrium than lanthanum is.
Conclusion
On every count, as demonstrated, La in group 3 is the true disruptive agent, obscuring chemical relationships throughout the elements and destroying compelling parallels between the two transition blocks currently known (d and f). It destroys:
- the parallel in basicity (Al-Ga and Y-Lu are both throwbacks becoming less basic immediately after the contraction; whereas Zr and Hf are almost equal, weakening the pattern);
- the parallel in double periodicity, in which the half-filled and fully-filled elements show the properties expected by high-school chemistry of them and that we know from high-school chemistry are those of Mn and Zn: Eu and Yb do that, Gd and Lu don't;
- the idea that similar elements should stay together when possible in the periodic table, because Lu is far more like Y than La is, and Lu is far more like the early d elements than La is; meanwhile, La fits so much better as an f element than as a d element;
- the idea that all elements have equal rights at the table, because it is not about nuclear properties, in not listening to lawrencium crying out that it fits so poorly with the late actinides;
- the whole point of calling the lanthanides lanthanides, by taking away from them the element that is most like lanthanum: lanthanum itself;
- consistency with aluminium, with thorium, with magnesium, and with palladium, in terms of how chemical differences are treated, instead going for the principle of mal so, mal so;
- and understanding of the really important difference in elements' aqueous chemistry: which elements' cations have pKa so low that they cannot persist in water. In this one case where, due to the restriction to water alone, a real sharp boundary actually exists, it is ignored. Meanwhile, artificial boundaries are erected with aplomb in its place to justify rupturing group 3 from group 4.
La under Y is just a historical fossil of the "asteroidal hypothesis" that only leads to a mistaken understanding of the real chemistry of these elements and unweaves the "rich tapestry of chemical relationships", as I have explained above and in many other comments. There is no compelling reason to carry on with it. Double sharp (talk) 07:28, 8 July 2020 (UTC)
APPENDIX
Google image search
Let's conduct a Google Image search and look at the first gross real full 18- or 32- periodic tables (without mistakes) that come out. The keyword I have chosen is "periodic table": I trust this is irreproachable. (As for the choice of 144 rather than 100, I plead high divisibility. ^_^)
Let's do a tally.
| Sc-Y-La | Sc-Y-Lu | Sc-Y-* |
|---|---|---|
| 22 | 20 | 102 |
| 15.3% | 13.9% | 70.8% |
I've been biased against the Lu table when counting. This, for example, could be argued to be a Lu or a * table. I have counted it as a * table. My criterion has been basically the footnote: La-Lu footnote means *, Ce-Lu footnote means La, La-Yb footnote means Lu.
I also admit that it is very possible that I have made mistakes in counting. However, the dominance of the * form (with a 2/3 supermajority, even) is a conclusion that is so strong that I think that even some mistakes in counting will not change that conclusion.
So much for the dominance of the La table. The dominant one is in fact the one that most unabashedly follows the old fossil that is the "asteroidal hypothesis", and that IUPAC is not even considering as a final option, even if it currently shows that as a compromise. If we ignore that, then the gain the La table has on the Lu table is pretty much insignificant.
But you should try doing it too, since Google search results may be localised. Double sharp (talk) 08:20, 8 July 2020 (UTC)
- @Double sharp: It appears that search results are localized, but the pattern is the same. With my sample of 144, this is what I found:
Sc-Y-La Sc-Y-Lu Sc-Y-* 28 21 95 19.4% 14.6% 66.0%
- The Sc-Y-* form again dominates by a large margin. And through crude statistics, one would find that the difference between Sc-Y-La and Sc-Y-Lu is indeed insignificant, and consequently neither clearly dominates. ComplexRational (talk) 00:51, 9 July 2020 (UTC)
- @ComplexRational: Hi! It's good to finally see someone else participating in this megathread again. ^_^
- As we can see from these results, the idea of a "chemistry battleship" flying the flag of the La table is extremely questionable. The average student is likely to be as familiar with the Lu table as the La table, judging by their similar frequencies. The dominating form is, in fact, the * table which cannot reasonably be expanded into 32 columns (unless you stretch the boxes of Sc and Y), doesn't make any chemical sense as Jensen noted (treating the f elements as degenerate d elements, which works extremely badly for, say, uranium), and is not even going to be considered as a final form by IUPAC.
- Therefore, there seems to be nothing policy-based against a proposal to switch to Lu at the present time. La does not dominate it and * is due to be deprecated officially. The proposed Lu form has an august history in the literature stretching back even to the 1890s. It has the support of the majority of those who examine the issue. And the scientific arguments are all on its side. All that remains is to start an RFC. Double sharp (talk) 09:11, 9 July 2020 (UTC)
- @Double sharp: Being the megathread it is, there is a lot of information to insider and I've been trying to focus on the most important bits and pieces. I am more convinced by the Lu arguments I've read, and given the support of the scientific community and lack of consensus against it here (no "battleship" as you describe), I'll join the RfC once it's underway. ComplexRational (talk) 12:30, 9 July 2020 (UTC)
It's nice to see your participation ComplexRational.
I doubt the Google image search could be regarded as a reliable source.
The result will be unduly biased by the false impression that the periodic table used within IUPAC is an officially-endorsed periodic table. In fact, IUPAC have made it clear there is no such thing as an IUPAC approved table. The one they have on their website is instead the one they use "within" IUPAC.
IUPAC project team survey findings
(continuation of prior post) The IUPAC project team's survey of chemistry text-books (which I contributed to) shows the La-Ac form as the most common by a 3:1 margin compared to either Lu or *. Double sharp, you suggested the survey data has been misinterpreted and that many tables showing La* could instead be interpreted as La-Lu. That's wishful thinking. The survey categorised tables as having a either 15-element wide f-block, or a 14-wide block starting with either La or Ce. That's all. Interpreting an author showing a table as La* and *Ce-Lu as meaning they rather intended this to be read La-Lu is lame.
No, the 15-column wide form is not due to be deprecated officially. The IUPAC project team's remit is to make a recommendation for either La or Lu. This will then go out for consultation to the chemistry community. IUPAC will then decide the next step. They may or may not accept it. If they don't then the status quo prevails.
A huge consideration is that IUPAC don't mandate the composition of groups. Why then would they be interested in mandating a form of periodic table? Don't confuse the fact of the IUPAC Group 3 project's establishment with IUPAC's endorsement of the outcome of the project. At the very least the project can be expected to publish some very interesting research and conclusions.
The policy basis for La is its predominance in the literature.
No, there is no such "given the support of the scientific community".
No, the majority who examine the issue don't examine the issue. Rather they usually examine one property. They only person who attempted a comprehensive examination of the issue was Jensen, and he was criticized by Scerri (chair of the IUPAC project group), for being too selective in his arguments. There was Holden (1985) who looked at seven properties but estimated the argument of Landau & Ligshitz (1958) to be the strongest. I've pointed out the shortcomings of L&L earlier.
No, the scientific arguments are not all on its side. There are still plenty supporting La, as set out in our IUPAC submission (which Double sharp has disavowed himself of). There will be plenty more in my article, which has been accepted for publication in Foundations of Chemistry, following three peer reviews. Sandbh (talk) 03:12, 10 July 2020 (UTC)
- @Sandbh: So here you describe the research question as: "f-block is 15 columns, or 14 columns from La, or 14 from Ce". Now my question is: is this the same statement as "group 3 are the ... elements not in this f-block" (i.e., complementary)? Or, in other words, is this another boiling down of the "group-3 discussion" (by your argumentation obviously)? And if so, what does this say for the "15-column f-block" wrt group 3 composition? Possibly, the answer is in the discussion already, so I'm sorry for asking something already clear ;-). -DePiep (talk) 10:02, 11 July 2020 (UTC)
- @DePiep: As far as I can remember this has not been discussed, mostly because the Sc-Y-* form hasn't been seriously considered by either side as an option.
- As for myself, I strongly support the idea that the f-block has 14 columns running from La, and that group 3 has Sc, Y, Lu, Lr (only d-block elements). So something like my userpage periodic table. I hope that meets your logical standards. ^_^ Double sharp (talk) 11:56, 11 July 2020 (UTC)
- @Sandbh: So here you describe the research question as: "f-block is 15 columns, or 14 columns from La, or 14 from Ce". Now my question is: is this the same statement as "group 3 are the ... elements not in this f-block" (i.e., complementary)? Or, in other words, is this another boiling down of the "group-3 discussion" (by your argumentation obviously)? And if so, what does this say for the "15-column f-block" wrt group 3 composition? Possibly, the answer is in the discussion already, so I'm sorry for asking something already clear ;-). -DePiep (talk) 10:02, 11 July 2020 (UTC)
- @Double sharp and Sandbh: I note that both tallies (DS image google, S chemistry text books)
return a similar overall result, circa 4:1:1.Precision/uncertainty is well within this huge spread (i.e., not significant). However, I do doubt the intention and interpretation of such a research. I don't think these sources (publication of a periodic table form) do represent any scientific statement. It can only be a statement when specific claims/statements are added to it; otherwise it is just a reproduction of an other source. These lists are not like this list. Even worse, a publication may be biased towards a preference for more simple or elegant or 'by authority' (IUPAC) form, which is a beauty contest not science. I understand Sandbh does argue for such criteria seriously, but that still does not convince me—FWIW. -DePiep (talk) 10:30, 11 July 2020 (UTC)- @DePiep: They return different results. The one Sandbh links to is supposedly 4:1:1 for La:Lu:*. Of course, it presumably counts tables with "La*" under Y as La, when you'd note they literally mean *. Mine gives 1:1:4 for La:Lu:* instead. (I guess you could argue the same thing, in which case the dominance of the * form goes even further up.)
- I don't think you can get much real science out of this, indeed. I did it to show that the idea that the La form dominates is false, and therefore that we have to look at actual science to do it. Like you, I am not convinced by arguments from authority one bit, especially when the "authority" is this divided, and the authorities who analyse the issue typically plump for Lu (regardless of whether Sandbh takes them seriously; it's another double standard, since some of the arguments he refers to for La are equally one-property arguments). Double sharp (talk) 11:56, 11 July 2020 (UTC)
- Big oops here, they are not the same indeed! I struck. Will have to revisit my thoughts on my first statement, not the 2nd though. -DePiep (talk) 12:18, 11 July 2020 (UTC)
@DePiep and Double sharp: The method used to count the types of table in chemistry textbooks was the same as that used/published in the Journal of Chemical Education article from 2008. That is to say, does the table have a 15- or 14-column wide slab under or associated with the main (spd) body of the table. A 15 counts as *-*. A 14 is counted as either La-Yb, or Ce-Lu, as applicable. For the 193 books the distribution was: 130 = 14 Ce-Lu; La-Yb = 30; *-** = 33. The Google image searches are plagued by bias arising from the false impression that the IUPAC *-** table is “official”, and unreliable sources. The IUPAC project’s survey of the literature, as per the JChemEd article, is sufficiently scientific, being based on reliable sources. Sandbh (talk) 13:27, 11 July 2020 (UTC)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac† | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| †Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||
- In other words, a table looking like the one to the right gets counted as Ce-Lu. I trust DePiep will soon come over to explain why that's wrong. ^_^
- So, just ask yourself how many periodic tables there are in the world. Then ask yourself how many appear in textbook flyleafs. The periodic table is such an icon of chemistry that it no doubt appears absolutely everywhere.
- And then maybe also ask yourself how much an article that actually sets out to examine the issue, even if you don't like that it presents one point (which hilariously seems to mean that if an author copies and pastes many other people's arguments into one paper, the whole apparently becomes more useful than the sum of the parts despite nothing having been added), is worth compared to a flyleaf periodic table presented with no justification for why it shows what it shows. Double sharp (talk) 13:46, 11 July 2020 (UTC)
@Double sharp: A table like the one looking to right, as the simplest explanation, gets counted as Ce-Lu.
For example, looking at some of the books in the IUPAC project survey:
- Kneen, Rogers & Simpson, Chemistry: Facts, patterns & principles (1972) show an 18-column La-, Ac- table. On the next page is the table labelled by blocks. Sure, enough, the f-blocks runs from Ce to Lu.
- Lee (1996), in Concise inorganic chemistry, 5th ed., shows an 18-column La↓-Ac↓ table. He later refers to the lanthanons or lanthanides as the f-elements Ce-Lu, including the preceding d-block element, La.
- G&E (2002) show La*-Ac*. They later label Sc-Y-La-Ac as d-block, and Ce-Lu as f-block.
And so on. Sandbh (talk) 08:27, 12 July 2020 (UTC)
Several authors have commented to the effect that La-Ac remains the most popular form. Mathias (1969) grumbled about it. Myers, Oldham and Tocci (2004, p. 130) found La-Ac was the most popular form of periodic table, a sentiment echoed by Clarke and White (2008)*; and Lavelle (2008; 2009). --- Sandbh (talk) 08:27, 12 July 2020 (UTC)
- *sample size = 27; La-Ac: *-**: Lu-Lr ratios were 1.61: 1.34: 1.0
| “ | @Sandbh: I object (strange talkpage flow, btw). The meaning does *not* depend on the content of the "footnote".
For starters: here the * does not mean "footnote" (like a note) as we undestand in academic literature. The * here, in the PT, is a placeholder for a part that is placed elsewhere. As such, they are just a replacement for a displaced part, they do not refer to some additional clarifying note. They are part of the graphic. There is no "meaning" in there. The modern word for this may be 'proxy' (for "100% resentative"). Simply, here: when it is below columnheader "3", it is a "3". Even when there by replacement placeholder. A pity this clarification must come back again and again. But if needed, so be it. -DePiep (talk) 02:02, 2 April 2020 (UTC) |
” |
@DePiep and Double sharp: The interpretation you're referring to, where La-Lu are considered to be part of group 3, is called the asteroid hypothesis and dates from 1881.
The only cases I'm aware of, of this interpretation, are yourself, John Denker, any maybe one chemistry text-book.
Jensen, who is a member of the IUPAC Group 3 project, has said of this interpretation:
- "I can hardly believe that a modern inorganic chemist would advocate such an antiquated interpretation of these elements, unless, as noted above, they have lost all contact between the underlying premises of their periodic table and the facts of chemistry."
It results in the entirety of the f-block being included in the d-block, an outcome which Thyssen & Binnemans, who wrote a long article on accomodating the rare earths in the periodic system, said one could not agree to.
Double sharp referred to the asteroid hypothesis as an old fossil.
In an Lu table, the asterisk/s will need to appear with barium and radium, thus Ba*-Ra**. I don't believe this would be interpreted as meaning the lanthanoids and actinoids will belong to group 2.
I agree with Jensen; Thyssen & Binnemans; and Double sharp. Sandbh (talk) 13:39, 12 July 2020 (UTC)
- Sandbh attacks the correctness of the interpretation Sc-Y-*-**, never mind that the dispute is actually on whether the periodic table above is actually saying that. Maybe I should call that a "conflationary fallacy". ^_^
- In a Lu table, there is no need for the asterisks to appear with barium and radium. They will appear in a gap between Ba-Lu and Ra-Lr, as they do in Droog Andrey and his colleague's periodic table poster, or as below.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | * | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| * | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | ||||
| ** | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No |
- Not the slightest logical problem. Putting the asterisks as Sandbh describes, in the same columns as Ba and Ra, will however lead to a lecture from DePiep telling you that that means that the Ln and An are in group 2 according to such a table because the asterisks appear in a column labelled 2.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba* | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | ||||
| **Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No |
@DePiep: For your confirmation if I have represented your opinion correctly. Double sharp (talk) 13:56, 12 July 2020 (UTC)
- @Double sharp: This one of yours is a type [6](d) argument: Omnibus obfuscation by unjustified reframing i.e. taking or reinterpreting my arguments out of context.
- No, I did not "attack" the correctness of the interpretation Sc-Y-*-**. Rather, I "attacked" the interpretation of Sc-Y-La*-Ac** as denoting a 15-element wide f-block. I interpret Sc-Y-*-** as denoting a 15-element wide f-block. Actually, no interpretation is required since it's obvious from the width of the "f-block". Sandbh (talk) 03:48, 13 July 2020 (UTC)
- Go talk to DePiep. It's his argument, not mine. He may easily see what you really did from you quoting Jensen, Thyssen & Binnemans, and me about the idea of all fifteen lanthanides under Y being bad, rather than addressing whether the asterisks being in the column marked "3" means that the table is saying that all fifteen lanthanides are under Y. Other than saying "I don't believe this would be interpreted as meaning the lanthanoids and actinoids will belong to group 2" (if it went Ca-Sr-Ba*-Ra** like my second example above), which is not an argument. Double sharp (talk) 04:09, 13 July 2020 (UTC)
@Дрейгорич: For your amusement commentary. Double sharp (talk) 04:11, 13 July 2020 (UTC)
@Double sharp: Good one. You summon DePiep and make an interpretation on his behalf. Then when the going gets tough, you bail out, and hand the burning pile to User:DePiep. You think it is funny and pass it on to Дрейгорич for his amusement. So much for civility.
@Дрейгорич: Your laughter from the sidelines is not welcome. Sandbh (talk) 10:41, 13 July 2020 (UTC)
- Nope. I quoted him exactly above. And you never addressed what I pointed out, quoting DePiep, so the going hasn't gotten tough for me at all. I also bet you that he'll confirm exactly the interpretation I made on his behalf, following his quote.
- It's also rather interesting to be lectured to about civility from someone who has used even more iconic rhetoric:
| “ | If you believe that I have a bridge to sell you. ... Sandbh (talk) 05:11, 14 May 2020 (UTC) | ” |
| “ | I have certainly not gone farther than you who referred to my logical argumentation as "fogging", "flat-earthing"(!), and other charming epithets. ... Double sharp (talk) 03:37, 15 May 2020 (UTC) | ” |
- But, very well. You have a point, I will not stoop to that level from now on. So, changed "for your amusement" to "for your commentary" above. Double sharp (talk) 11:50, 13 July 2020 (UTC)
Group 3 article (in press)
(continuation of prior post)
- That article, of course, being the one that in its original version you brought here for comments. Meanwhile pretty much every argument for La has been refuted to the complete satisfaction of all participants but you, as evidenced by the rather lopsided consensus above. And all the issues examined by the Lu proponents fit together in one simple logical narrative as Matthias has so nicely explained for us in 1969, see below. All La has going for it is a chemically irrelevant accident in ground state gas-phase electron configurations.
- I guess I shall have to summon DePiep again to explain for the nth time why putting "La*" in one cell below Y indicates a * table. Double sharp (talk) 03:23, 10 July 2020 (UTC)
@Double sharp: The article has been revised since you saw it. It matters to me what you and the other participants here think, since what you and they think makes me think hard, and has made an impact on the MS (and will probably do so until I sign off on the proof copy) and that is tremendously good. The article has benefitted enormously from this process, thank you all.
At the end of the day I have:
- for the first time, to my knowledge, stepped back from the minutiae of physical, chemical, and electronic properties and explored considerations of regularity and symmetry; natural kinds, and quantum mechanics;
- included ten interlocking arguments, in the context of a chemical periodic table;
- in so doing, sought to demonstrate a new way of thinking about this matter;
- recast the last of my ten arguments as a twenty-word categorical philosophical (viewpoint-based) statement;
- 108 supporting citations; yes, I know, these must all be wrong, of course ^_^
- survived and benefited from three peer reviews (47 change/clarification requests); and
- been accepted for publication by Scerri, editor of FoC, (and also chair of the IUPAC Group 3 task group).
That'll do me. Sandbh (talk) 06:13, 10 July 2020 (UTC)
Lack of support?
(continuation of prior post)
- @Sandbh: And does it not make you wonder that absolutely nobody here agrees with the La option on its own merits? Even R8R has said that if he was writing a book for himself he'd be very fine with the Lu option. Double sharp (talk) 06:34, 10 July 2020 (UTC)
- @Double sharp: No. I remember what happened the first time we switched to Lu. That was on the basis that we thought we knew better and, certainly for me, because I thought the 32-column La form was ugly. FJ rightly, albeit inappropriately, scolded us. Since then I know more about the philosophy of chemistry, and the way Scerri looks at it, and I’ve learnt a lot from Philip Stewart, the polymath, and a few other clever people like Eugen Schwarz. I’ve never really stopped thinking/reading about it and how Scerri has been trying to address it from a philisophical POV, and many of his responses to my previous suggestions.
- Absent this background, it doesn’t surprise me others favour or are inclined to Lu, since I would’ve counted me among those too.
- This doesn’t necessarily mean I’m right. Mendeleev experienced the same frosty reception. Whereas he had something new, I having nothing new to offer except perhaps a few new observations and a harmonised set of arguments. Sandbh (talk) 10:29, 10 July 2020 (UTC)
Mendeleev's predictions
(continuation of prior post)
- @Sandbh: Funnily enough, the reason why pretty soon nothing was said against Mendeleev is that he made scientific predictions. Greenwood and Earnshaw give a table on p. 217 (2nd ed.) even for eka-aluminium / gallium.
Comparison between Mendeleev's 1871 predictions and the known properties of gallium Property Mendeleev's predictions Actual properties Atomic weight ~68 69.723 Density 5.9 g/cm3 5.904 g/cm3 Melting point Low 29.767 °C Formula of oxide M2O3 Ga2O3 Density of oxide 5.5 g/cm3 5.88 g/cm3 Nature of hydroxide amphoteric amphoteric
- Those are self-evidently falsifiable. Indeed, Mendeleev even put it to the test. Let me quote the gallium article (I brought it to GA back in 2016):
| “ | Originally, de Boisbaudran determined the density of gallium as 4.7 g/cm3, the only property that failed to match Mendeleev's predictions; Mendeleev then wrote to him and suggested that he should remeasure the density, and de Boisbaudran then obtained the correct value of 5.9 g/cm3, that Mendeleev had predicted exactly. | ” |
- Despite your words "This doesn't necessarily mean I'm right", let's see how you reacted to me asking you what could possibly falsify your stand:
| “ | Well, if Scerri, who is a great fan of Popper, knew the answer it would've been pens down. Likewise, I don't know the answer, honestly. But I'd know if I saw it; I haven't seen it yet. This is not a logical proof exercise. There is no logical provable answer. Chemistry, with its qualitative and quantitative aspects is like that: As Poliakoff (2011) said:
And there is this:
To me, you and Droog Andrey don't appear understand that. For DA, this is is surprising. Chemistry is full of ambiguities and fuzzy boundaries. Logic doesn't apply in this case, only one's strength of arguments. |
” |
- Never mind, of course, that how one is supposed to gauge "strength of arguments" if "logic doesn't apply" is beyond me. Or probably anyone else, really. But, of course, feel free to go around talking to chemists and seeing what they think of the idea that "logic doesn't apply" to their science. And maybe ask yourself how you can have any arguments at all without the logic that you've been discarding as irrelevant whenever I invoke reductio ad absurdum. And maybe this gem ought to be quoted as well:
| “ | @Droog Andrey: Not all arguments in chemistry are based on logic. "In many respects, computational chemistry is still an art, and relies upon a delicate mix of physical intuition, pragmatic cleverness, and practical know-how." Sandbh (talk) 13:11, 16 May 2020 (UTC) | ” |
Logic, harmony & the basis of periodicity
(continuation of prior post)
- Well, go and integrate . (*) You clearly need a bunch of intuition, pragmatic cleverness, and practical know-how to figure out what to do with this. Once you figure it out, you may go forth and tell the mathematicians too that not all arguments in their subject are based on logic. Let's see how that goes. ^_^
- And guess who here actually has a harmonised set of arguments that explains the whole thing very nicely starting from chemically relevant 4f involvement on La. In one stroke that explains (1) melting points; (2) superconductivity; (3) high coordination numbers; (4) cubic complexes; (5) crystal structure; (6) heat of sublimation. All we have from you are arguments that are artificially limited to stop them from leaping at each other's throats. My idea of "fuzzy configurations" is not new, since that's all standard knowledge among actual chemists who understand what electron configurations really mean, but that's great since it means it can actually be used on WP since it's not even original research.
- (*) Solutions welcome from anyone reading this on my talk page. Any and all approaches encouraged. For more challenge, replace the top limit by . ^_^ Double sharp (talk) 10:51, 10 July 2020 (UTC)
Meanwhile: stepping back from chemical, physical, and electronic properties means stepping back from the very properties that are used as the basis of periodicity. It is these properties that the periodic law states are a periodic function of atomic number. Nice going. Although it does have the beneficial side effect that this means that the whole argument "but La has no 4f electron in the gas phase ground state" has to be thrown out too by consistency. Judging by the conversation we appear to be having here, I would guess that that has not been done, because it seems to favour La.
Symmetry and naturalness
(continuation of prior post) Meanwhile, it's self-evidently obvious that a Lu table is more symmetric and regular than a La table. Remember, by consistency throwing out electron configurations means the 4f thing doesn't even have to be refuted. Talk about unwanted consequences. Even if we put them back, of course, in chemical environments La 4f is just as real as Th 5f which we agree exists and is significant. And the Lu table gives more natural blocks in terms of natural kinds, because Lu is far more like a transition metal than La. But of course, since this supports Lu, it will be dismissed as something not to lose sleep over. Complete with a question about why chemists should care about what mathematicians generally call reductio ad absurdum, never mind that this isn't just a thing in mathematics (which, as any chemistry student at university will tell you, is extremely important for chemistry), but also one in logic. So, just try telling any chemist that logic is irrelevant to his or her discipline, and see if he or she is not insulted.
Erasers and the irrelevance of (classical) logic
(continuation of prior post)
Well, let me end off paraphrasing what Droog Andrey said. A scientist uses paper, pencils, and erasers. Philosophers don't even need erasers. So far he and I have been the eraser salesmen here. Somehow everyone else seems to recognise the need for our wares but you. Maybe you should ask yourself why that's so. It can only improve your arguments to analyse why others are completely unconvinced and try to address the problem.
But, since logic has been declared irrelevant, this may well be pointless. Of course we've already seen the requirement of falsifiability being ignored too, so this is just a confirmation by now. So, see you at the RFC. ^_^ Double sharp (talk) 06:50, 10 July 2020 (UTC)
- @Double sharp: You've largely hit upon what I was going to say. There is a significant presence of fuzzy logic in chemistry, which I feel you overlook in preference to B/W logic. That's where Schwerdtfeger, Smits & Pyykkö (2020) were coming from. Our thrust-parry-thrust cycle unnecessarily arises from applying B&W logic to fuzzy logic considerations. There is an analogy to asking which elements are metalloids? While the answer is fuzzy it’s still possible to discern which elements are most commonly recognised as metalloids. Thus, you assert Sb is a metal (which is fine, in the particular context you have in mind); whereas Sb turned up in 87% of the 194 metalloid lists I looked up.
- The formidable and long-standing environmental characteristics at play are:
- predominance of the La form (analogy to metalloids);
- conservatism of the chemical establishment (pointed out to me by Scerri);
- the highly successful reliance on free atom configurations;
- the Lu form offers nothing astonishing enough to replace the status quo;
- the split d-block is not seen in the 18-column form;
- significant fuzziness in chemistry/qualitative-quantitative mixture;
- intermittent one-trick pony arguments for Lu have appeared over, say, 60+ years and effectively nobody could care, even when Jensen tried herding them;
- Lavelle and the "silent majority"; Restrepo (2019); Liu et al. (2019);
- The La form has not become less than useful in the context of what Jones’ asserted: "As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics."
- The deck is hugely loaded against the Lu form other than in specific contexts e.g. the Janet form, with its focus on regularity and symmetry. Sandbh (talk) 02:25, 11 July 2020 (UTC)
- And that's why discussion of this with you is fruitless. Because when it comes to the La form, you refuse to analyse it. The La form, as shown above, hardly has "predominance". (Unless this is some new definition of the word in which you don't even need a frequency of 20% to be "predominant".) The chemical establishment is not so conservative that it couldn't get rid of Be-Mg-Zn and B-Al-Sc. Already today H-F-Cl is a rarity; only H over Li and floating H are in any sense commonly encountered. I and Droog Andrey demonstrated, referring to numerous reliable sources, exactly why "reliance on free atom configurations" is not going to give you a good understanding. And we demonstrated how the Lu form ties together numerous things about the blocks into one coherent whole. The 18-column argument is basically saying that you can hide something by sweeping it under the rug. And, as I noted in Archive 44, somehow equally one-trick pony arguments for the La table (e.g. Restrepo) are accepted by you, but never when they're for the Lu table. And, as I noted above, the very idea that the chemical establishment is for La is questionable (that must be why IUPAC, and the majority of periodic tables people see, are *, of course). And we demonstrated that in fact the La table opens a can of worms that creates lots of hard cases: how to refute H-F-Cl, Be-Mg-Zn, B-Al-Sc, C-Si-Ti, Ca-Sr-Yb, Ti-Zr-Ce-Th, Ti-Zr-Hf-Th, V-Nb-Ta-Pa, Cr-Mo-W-U, etc. But, of course, the only argument that we ever get back is "but nobody loses sleep over it". So, in other words, we must keep running with the tradition. Even if it is not a tradition and most people who examine it conclude that it is wrong anyway. Wow.
- Something R8R said in Archive 42 is quite revealing:
| “ | (I imagine here a cowboy that shoots from a pistol, runs out of ammo, drops the pistol, takes out another pistol, and keeps shooting as if nothing had happened. However, a whole lot has changed between those two lines of argumentation.) --R8R (talk) 19:54, 18 March 2020 (UTC) | ” |
- But this is more like a cowboy who shoots from a pistol, runs out of ammo, and keeps shooting because that's the predominant pistol he used to fire his bullets. Never mind that a new situation has asserted itself: the pistol doesn't work anymore.
- Incidentally, note that all these bullets only matter when they support the La argument, for you. The moment thorium is mentioned, let's see how fast you run away from that "highly successful reliance on free atom configurations" to save the La table. Just like what Jensen said about Lavelle.
| “ | When it comes to the question of why La and Ac should remain in the d-block rather than being reassigned to the f-block, Lavelle offers no new chemical or physical evidence other than his constant reiteration of the fact that both elements contain d-electrons in their ground-state valence configurations, but no f-electrons. Yet in the cases of both Lu and Th, for which this is equally true, he proceeds to inconsistently argue that this fact is of no consequence when it comes to assigning them to the f-block. As with the case of the revised configuration for Lr, which counts when it comes to not placing this element in the d-block but is irrelevant when it comes to placing it in the f-block, this arbitrary and naive use of electron configurations, to the exclusion of all other evidence, is logically inconsistent and leaves one with the impression that the only true argument that Lavelle has for the major premise of his diatribe is that La and Ac should remain in the d-block because that is where IUPAC places them in its official periodic table [which it doesn't, so you'd have to substitute "Authorities So-and-So who actually don't analyse the situation"] and therefore all rational discussion of other possibilities is strictly forbidden. | ” |
- All these bullets also apply exactly the same to Copernicus in 1543. As a matter of fact they would still apply even later, when heliocentrism was on the rise but there were still many traditionalist geocentrists. Just make the obvious replacements.
- I think that says it all. For everybody else at least, who recognises the need for logic to say anything useful about the world. Double sharp (talk) 03:38, 11 July 2020 (UTC)
@Дрейгорич: Exhibit K. Double sharp (talk) 04:33, 11 July 2020 (UTC)
- Soon, we'll need an Exhibit Z or something. ― Дрейгорич / Dreigorich Talk 05:08, 11 July 2020 (UTC)
- @Дрейгорич: Most of this talk page already is, to be fair. From the viewpoint of logic, most of what Sandbh is saying seems completely unsupportable. From Sandbh's viewpoint, of course, nothing can supplant La. But note that exactly what the Lu authors seem to be guilty of, according to Sandbh, keeps changing. You saw Exhibit G already, I suppose.
- But let's look at how seriously he takes his own idea that "reliance on free atom configurations" is "highly successful". Because that will make a very fine Exhibit L.
| “ | Sticking with blocks alone. That is what most chemistry text-book authors do. They then drill down into the electronic filling sequence, and present the table as Sc-Y-La because it’s not until Ce and Th where f- electrons first make their presence felt. (An objection can be raised to Th d2s2. Still, the presence of ~0.5 of an f-electron is indicated in the solid). And we know the split d-block doesn't become over-visible due to the predominance of the 18-column form. ... Sandbh (talk) 06:12, 8 July 2020 (UTC) | ” |
- This is pretty funny. So, apparently reliance on free atom configurations is not so successful after all for thorium. Since the only thing he can come up with apparently is "the presence of ~0.5 of an f-electron is indicated in the solid", i.e. not-so-free atom configurations.
- For most people this generally would be considered an inconsistency. Quite possibly also a direct contradiction. Not for Sandbh, apparently.
- But it gets even better, because the little problem with that is that lanthanum also has some f-electron presence indicated in the solid. Glötzel showed this for fcc La (~0.17 of an f-electron), which is the metastable state at standard conditions. And there is also Wittig to explain it to us. f electron involvement in La explains anyway its (1) melting point, (2) heat of sublimation, (3) crystal structure, (4) high coordination numbers, (5) cubic complexes, (6) superconductive properties, and (7) makes immediate parallels to many parts of the periodic table (the contractions, the typical delay for heavy elements, cf. Ac-Th-Pa, Lr-Rf, E121-E126). Just try explaining all seven together with La arguments. "Simplest sufficient complexity" indeed. XD
- Of course, whenever I brought it up in Archive 44, he dismissed it by quoting Wittig out of context. Wittig wrote "This result ends the speculation about a special "f electron mechanism"" for La, but then proceeded to go on in his very paper to propose another different f electron mechanism for it, because the elephant in the room that is La's anomalously high critical temperature remains unexplainable if La was a totally normal d element. Conveniently, Sandbh did not quote that later bit.
- When it came to 4f involvement in Lu, which has no support whatsoever, he then invoked the absence of anyone rebutting it: "I continue citing 4f in Lu in the absence of a Wittig-like statement saying "This result ends the speculation about a special "f electron mechanism".". Brilliant! I suppose hydrogen has 4f involvement as well, since no one felt the need to refute that either! And guess how far we've already gone from the gas-phase electron configurations, in which neither thorium, nor lutetium, have any f valence electrons! XD Double sharp (talk) 05:34, 11 July 2020 (UTC)
- Apparently hydrogen is an f-block element despite having no f electrons. LOL. ― Дрейгорич / Dreigorich Talk 06:02, 11 July 2020 (UTC)
- @Дрейгорич: Ah, I would be careful about saying that without qualification. Having no f electrons in the ground state cannot be prohibitive, as we can see from thorium [Rn]5f06d27s2. (That's what generally makes Sandbh's electronic arguments double standards.) What is prohibitive is having no involvement of f orbitals in chemical bonding whatsoever. Double sharp (talk) 08:32, 11 July 2020 (UTC)
- Je suis un idiot. Either way, hydrogen fails both. ― Дрейгорич / Dreigorich Talk 09:13, 11 July 2020 (UTC)
- @Дрейгорич: Ah, I would be careful about saying that without qualification. Having no f electrons in the ground state cannot be prohibitive, as we can see from thorium [Rn]5f06d27s2. (That's what generally makes Sandbh's electronic arguments double standards.) What is prohibitive is having no involvement of f orbitals in chemical bonding whatsoever. Double sharp (talk) 08:32, 11 July 2020 (UTC)
- Apparently hydrogen is an f-block element despite having no f electrons. LOL. ― Дрейгорич / Dreigorich Talk 06:02, 11 July 2020 (UTC)
@Double sharp and Дрейгорич: I don’t understand my reference to free atom configurations being taken out of context. Then again, my arguments are regularly conflated or fallaciously extended so I should not be surprised.
Never mind Th. The fact remains that the electron configuration model, as an approximation, is highly successful.
Schwerdtfeger gets it. Smits gets it. Pyykkö gets it. The three referees who vetted my MS get it. Here it is again, in case you missed it the several other times I mentioned it:
- ”Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind." [italics added]
- ”Fuzzy concepts like chemical similarity often lead to unnecessary disputes [italics added] concerning the PTE."
Double sharp, your focus on Th is unfathomable to me. As is your seeming inability to understand those environmental factors.
On one-trick pony arguments, I said, and you ignored it, that Jensen attempted to draw the Lu ones together and his efforts failed to gain traction. Nobody has done that for the La arguments. Now I will have, when my article is published on line.
Yes, the La form is predominant per the IUPAC published data. Your interpretation of La* is astonishingly stretched. Even Mathis acknowledged the popularity of the La form.
That’s right, nobody loses sleep over it. Haven’t for the past 60 years. Schwerdtfeger doesn’t. Smits doesn’t. Pyykkö doesn’t. The three referees who vetted my paper didn’t. If you read this far go back to paragraph two of this response. Lather, rinse, repeat, as you say.
Your classical logic paradigm is misplaced.
No, the authority is not divided. Rather, per Jones, there is zero basis for change.
I’m not inclined to say any more about this, but we’ll see. Sandbh (talk) 13:47, 11 July 2020 (UTC)
- Unless you intend to sit out the RFC I'm planning to start in a couple of days proposing to reflect our amazing 7-1 consensus that something is wrong with some or all of your arguments, I strongly suspect you will be saying something more about it. I can't say I'm looking forward to saying anything more about this either, since I think everyone else here but you understands the need for a classical logical paradigm anyway, and just about every point has been exposed over 9000 times by now (obvious hyperbole). But needs must when needs must. I made a mistake when supporting La previously, and I am scientific enough to say "I take back everything I claimed before and state the opposite" when I learn information contradicting what I thought I knew, without having to have a IUPAC project tell it to me. With such a level of support for the Lu side on this page, it would be in fact rather perverse not to start an RFC.
- But thank you for so clearly disclaiming the need for classical logic. I must say that I've never actually seen anyone else outright saying that logic doesn't apply when arguing about natural science. It certainly goes a long way to explaining why we are at loggerheads on this issue and probably will continue to be so for the foreseeable future. Not to mention why this debate has been utterly fruitlessly going in circles between us two, but has been extraordinarily successful at convincing everybody else that your arguments aren't terribly convincing. ^_^
- Respectfully, Double sharp (talk) 13:54, 11 July 2020 (UTC)
Overall notes re APPENDIX
I need more time to respond carefully to all posts here. More so because I made a huge error in my initial post in thios section [1] ;-( . Anyway, for now I am not that far that I can join the cynicism Double sharp sometimes refers to. -DePiep (talk) 21:23, 12 July 2020 (UTC)
Lutetium (IV or V)
@Double sharp: What do you make of these references(?):
- [1] "…double-decker type, sandwich-like phthalocyanine multiether lutetium(IV or V) complexes, shown in Fig. 3(d)…" (p. 4564)
- [2a] "The analogous alkyl-substituted lutetium(IV) complexes (Table 4-19, R=C,,H2,,+1, n = 8, 12, 18) show a narrow Dhd phase (<13 °C)." (p. 164)
- [2b] "The sandwich-type bis(phthalocyaninato)lutetium(IV) complex bearing p-octadecyloxyphenyl groups displays a tetragonal columnar (Dmd) mesophase at room temperature and a hexagonal columnar (Dhd) phase at higher temperatures." (p. 170)
- Metallomesogens: Synthesis, properties, and applications
- [3a] "…lutetium(IV) diphthalocyanine (Pc2Lu)…" (p. 715)
- [3b] "Absorption spectra of a 4.8 × 10–6 M solution of lutetium(IV) diphthalocyanine in DMSO (0.5 mL 13 M KOH) (1) before and (2) after the addition of 4.8 × 10–7 M phenanthrene." (p. 718).
--- Sandbh (talk) 06:38, 7 July 2020 (UTC)
- @Sandbh: I strongly suspect that these are mistakes, or else a case of formal oxidation states being taken far too seriously. (Which is not at all implausible, since a hurriedly-written "III" with the Roman-numeral overbar and underbar can look a lot like a "IV".) The homologous YPc2 is known (10.1103/PhysRevLett.67.244): if it really involved somehow going beyond the stable octet in a IV oxidation state, we should've heard of it as something exploding the idea of the noble gas configuration being unable to be passed. One would also then wonder where Rb(II) and Sr(III) are, since Rb has a lower 2nd ionisation energy than Y's 4th ionisation energy. And one would similarly start wondering about Yb(IV) and Yb(V). (Also: seriously? Lu(V)? When even Pr(V) needs such extreme conditions to appear? The only explanation I can think of is that this is meant to be a formal oxidation state only that does not really correspond to grabbing electrons out of the buried 4f shell.) No: the article I gave the doi of notes, "the neutral green form of lutetium diphthalocyanine (LuPc2) has a one-electron ligand-oxidized "sandwich" structure in which the unpaired electron is located indistinctly on both phthalocyanine rings". If I understand this correctly, then it is certainly no more Lu(IV) than [Hg(cyclam)]3+ is Hg(III). (It's not, see 10.1002/anie.200802233.) In both cases it is simply that the ligand is redox-active; the metal has not been oxidised beyond its standard greatest oxidation state! Double sharp (talk) 08:14, 7 July 2020 (UTC)
@Double sharp: I tend to agree. It’s odd these things supposedly pass peer or editorial reviews, either as mistakes or sloppy terminology. Sandbh (talk) 09:48, 7 July 2020 (UTC)
Three cheers for Bernd T. Matthias
From Systematics of Superconductivity, a lecture in Superconductivity: Proceedings, Volume 1 (1969). Please excuse some datedness regarding the lack of f character expected for Ac and Th, but he gets La right.
| “ | Let me point out, however, that we made some changes in the periodic system. Here is Lu; all of your text books show La in its place. We just shifted the whole thing over and started La near Ba then all the rare earths, and finally Lu under Y. From all points of view, a crystallographic, metallurgical, superconducting, melting point in particular—this is the proper way to do it. And it was a mistake in the periodic system—unfortunately mostly propagated by the Welsh [Sargent-Welch?] Company, that La was here and again, everybody copied it. When we published that there was a mistake in the periodic system and La should be where Lu is and vice-versa, we were extremely proud of it until we found it in the Landau Lifschitz book where they had previously mentioned it. In subsequent papers we gave them credit but we didn't know it at the time we came to this conclusion.
... Let me now come back to the systematics of Tc. There are two cases on which I want to concentrate for a time. One is Sc, Y, Lu, La. Now, how do these elements differ from one another? If you believe superconductivity has something to do with resistivity (ρ), chemical valence (V), number of carriers (n), boiling point (b.p.), χ, density of state (N), θD, then we write Tc = Tc(ρ, V, n, (b.p.), χ, N, θD) If you do this there is no distinction between these 4 elements. But Sc, Y, Lu are not superconducting and La is at 6°K. Because it is superconducting, obviously there is something different. Now what is it? The largest difference is in the melting point since this temperature is radically different from what it should be. It is 500°K too low. And those are the only two pronounced features in which La differs but of course if we are trying to explain superconductivity we cannot use superconductivity as a criterion—this is like calculating the interaction constant by measuring the Tc. And only the melting point is the other independent parameter that makes La different from all the other three. A while ago we found that Mo was a superconductor at 0.9°K by cleaning, which was a rather unexpected feature. So we decided that maybe Sc, Y and Lu were also dirty. We tried to purify, and then we tried to extrapolate from alloys. There was no hope for Y to become superconducting above 10−2°K. That is when I realized that something was really wrong, because all these elements were identical and the one quantity in which they were not was the melting point. The reason for this turned out to be in the character of the electron configuration. As I showed you before, La is the last element before the rare earths begin, before the f electrons are actually localized. So in La you have the f electrons just about to appear (I am talking in pictures) but are not yet quite there; this is the cause of the superconductivity of La. And I will show you how analogous La and U are, strictly from every point of view—melting point, superconductivity, all other behaviours. The similarity between the two is actually quite astonishing. ... Now let me go on from yesterday where I said the melting point of La is too low because of the f electrons or the impending f electrons. Obviously the impending f electrons will interfere with the melting point. Everything in the periodic system always varies in a monotonic and symmetric fashion. For example, wen you go from left to right the melting temperature goes as in Fig. 6. However if you go vertically, the melting point is again a monotonic function. So in order to evaluate what the melting point of a certain element does we interpolate vertically and then horizontally and by doing this you get extremely precise data as to what you should have. For most of the elements this fits extremely well. And when I say to you La is too low then it is the fact that we get a melting point of 920°[C] and we should get a melting point of from 1500–1600°[C]. [Which is close to the 1663°C Lu has.] Why is this so? Well, as I told you before, all the electrons that contribute to superconductivity, to forming the crystal lattice, are the electrons outside the filled shells. However, there is one exception—f electrons which will not contribute because the f electrons are too far inside the electron shell and too close to the nucleus. For instance, the average radius of s and d elements in La is 1.8Å; the f electrons of the following elements (Ce---) have a radius of only about 0.9Å or less. So they no longer contribute to the formation of the crystal lattice. And what we say is this: while the normal trivalent element will have 3 valence electrons, namely s and d electrons, in the case of La it may look like this, (sd)3−x(4f)x, where x may even be time dependent if it's a virtual state. This mechanism is responsible for inhibiting the contribution to the formation of the crystal lattice and thus lessening its rigidity hence lowering its melting point. Clearly as we go on to the rare earths this effect will disappear as once there are no more f electrons because of the filled shell [Lu] then everything should be just as before and we should get the correct melting point. Now in Fig. 7 let me show you how this is born[e] out. Everything again is monotonic, La and Ce have the lowest melting point. Then the depression begins to disappear. The tendency to fill half-filled or filled shells is enormous. Therefore what happens in europium is that you pull one electron from the d range and turn it into an f electron. One is left with a divalent metal and one extrapolates to get the correct melting point for europium. In the case of Yb the same thing is repeated. Instead of the 13 f electrons according to the periodic system, you have 14 by making it a divalent element. This is the reason or the dips in melting point at europium and ytterbium. As we begin to fill up the f electrons the melting points become more and more normal and by the time we arrive at Lu everything is normal again, just as Sc and Y. So this so-called hybridization between s and d and f electrons is in my opinion the cause of the reduction in the melting point of La and the consecutive rare earths as Ce. Always remember whether you have a virtual f electron or an f electron that is localized; they will never contribute to the rigidity of the crystal lattice whatever you may find in the literature. Basically, there are no exceptions in the periodic system or in nature. As I've shown you in the periodic system before, the 4f's eventually repeat themselves in the 5f's, namely from U on. Now the 5f and 6d levels are much closer together than the 4f and 5d levels and this close proximity interferes more. That is one of the reasons, but essentially the 5f's will be more active because they are closer to the binding electrons, to the valence electrons. They will be more active in changing the behaviour from that which would normally be expected by extrapolation or interpolation. The results are in Fig. 8. This is just where I show you the discrepancies. The line is what you should get for clean cut trivalency in rare earths and you see how the discrepancy (the length of the line) vanishes. In Fig. 9 you see the same thing for the 5f's, and you see it is very intriguing. The same behaviour repeats itself only much more so. In protactinium the f character begins and gets more and more pronounced towards larger atomic numbers. The depression of the melting point is even more than anything ever expected before—it's huge. U in the periodic system is a 6-valence element. W has a melting point of 3300°C. U is [was!] below in the periodic system and melts around 1200°. It gets even more drastic with neptunium and plutonium, which are around 600°C. Neptunium, which is [was!] the same column, corresponds to rhenium which is about 3000°C. So we now get a discrepancy which is much, much larger yet. The discrepancy due to the 5f hybridization is just stunning and that, of course, is the explanation for why these elements—Np, Pu and also U—have these extremely low melting points whereas from their position in the periodic system you would think they would have had a high melting point. |
” |
I add: from chemistry, from electron configurations too, this is the proper way to do it! Not a single metric is in favour of the table with La under Y and nothing else changed. Everything relevant points to Lu under Y!
And what I have kept on insisting, that the fact that La precedes the total collapse and doesn't have an f electron in the ground state is exactly why it has the biggest f involvement of the lanthanides (together with Ce), that the analogy means Yb is the fully-filled shell, is totally vindicated.
There is only one thing I want to add to explain something here. Why is the melting point depression higher for Ce than for La? Because for La 4f has not fully collapsed yet, it instead acts as a directly valent orbital. It has collapsed enough to be non-hydrogenic but not be drowned like 4f is famous for being; that is part of why it has been so misunderstood. For cerium 4f is more collapsed, it is slightly more indirectly valent, and you get a bigger melting point decline. This one is understood better. But after that, from praseodymium onwards, the gap is smaller, even though 4f is yet more collapsed and almost totally indirectly valent most of the time. Past neodymium you cannot get the +4 state, except for terbium because of the half-filled shell. Why? Because now the gap is so big that the hybridisation becomes more and more energetically infeasible. It is the same reason, why the depression is there for Ac vs Lu, and Th vs Hf, but from Pa onwards it becomes incredible. And then it stops: americium, curium, we go back to trivalency. But then berkelium, californium, einsteinium, we are going on the border back to divalency even. Now the 5f-6d gap has become even bigger than the 4f-5d gap, and you can see how nobelium melts at about the same temperature as ytterbium only.
All of this is totally regular, all of it is totally in keeping with the Lu table. The La table only gives a bad misunderstanding of the situation and has very poor explanatory power. Even for what looks like its greatest success, the general trivalency of the Ln, it turns out that this actually is not as directly related to the ideal configurations as the La table would have it. Double sharp (talk) 10:55, 9 July 2020 (UTC)
- Any theory from 1969 based on superconductivity should be treated with extreme caution, as you rightly note. I cannot find any citations for Mathias' lecture. Reference to "From all points of view" is unjustified unwarranted ebullient hyperbole. It is not necessary to invoke f electrons to explain structure and melting point. L&L are unhelpful. Mathias says, "Sc, Y, Lu are not superconducting and La is at 6 K." To my knowledge, the superconductivity status of Lu has still not been conclusively resolved. Mathias says group 1 and 2 metals are not superconductors whereas Be is. He said group 9 metals were not superconductors. Wrong. Rh and Ir are. Sandbh (talk) 07:40, 10 July 2020 (UTC)
- Just try explaining all the things f involvement in La explains as simply without it. The denial not only doesn't fit Occam's razor, it also means that the only explanation left for the cubic complexes of La is magic due to symmetry considerations. ^_^ Double sharp (talk) 11:22, 10 July 2020 (UTC)
@Double sharp: No magic is required. Here is Parish, once again:
- "Two features are particularly striking in the chemistry of the 4f-elements, viz. (a) the uniformity of the +3 oxidation state and the small number of other oxidation states, and (b) the irregularity of structures and occurrence of high coordination numbers. In the compounds described here, coordination numbers of six, seven, eight, and nine have been mentioned, and in other compounds ten- and even twelve-coordinate metal ions are found, eg. in La2(SO4)3.9H2O and (NH4)2Ce(NO3)6. It is tempting to think that the f-orbitals must be involved in the bonding, since the maximum number of hybrid orbitals which can be constructed from an s-p-d -set is nine, and to obtain eight bonding orbitals directed towards the corners of a cube (as in C6O2) requires at least one f-orbital. There is, however, no evidence to suggest that the f-orbitals are involved at all in the bonding, and even ligand-field effects are extremely small. All the compounds appear to be essentially ionic, with very little covalency involving even the 6s- or 6p-orbitals. The curious structures and high coordination numbers are similar to those found with other large cations (e.g. salts of Ba2+ or Pb2+) and are presumably a result of the optimisation of electrostatic forces. The large internuclear distances necessitated by the radii of the cations will cause the electrostatic energy per pair of ions to be relatively small, despite the high cationic charge, and many such pairs must be formed to achieve a sufficiently large lattice energy. Similarly, the large radii diminish considerably the polarising effect of the +3 charge which would otherwise be expected to lead to considerable covalency." (p. 151)
- --- Parish RV 1977, The metallic elements, Longman, London, p. 151
- When I quote something from 1969, it is dismissed as dated. Then Sandbh proceeds to quote something from 1977, which is also pretty dated, and believe it over a source from 2002 (quite a bit less dated) which he himself also knows and has referred to when asking about this:
"The one case in which contributions to the bonding from the f orbitals is possible is in complexes of the heavier elements in which the coordination number is high. Use of the s orbital, together with all the p and d orbitals or one valency shell, permits a maximum coordination number of nine in a covalent species. Thus, higher coordination numbers imply either bond orders less than unity or else use of the f orbitals In addition, certain shapes (such as a regular cube) or lower coordination number also demand use or f orbitals on symmetry grounds. These higher coordination numbers have only become clearly established recently, but their occurrence in lanthanide or actinide element complexes suggest the possibility of f orbital participation. Examples include the 10-coordinate complexes mentioned above, LaEDTA(H2O)4 and Ce(NO3)52- or 10-coordinate La2(CO3]3.8H2O; 11-coordinate Th(NO3)4.5H2O (coordination by four bidentate nitrate groups and three of the water molecules); and the 12-coordinate lanthanum atoms in La2(SO4)3.9H2O-with twelve sulfate O atoms around one type of La atom position."
— MacKay KM, MacKay RA &Henderson W 2002, Introduction to modern inorganic chemistry, 6th ed., Nelson Thornes, Cheltenham
- Brilliant! Double sharp (talk) 02:42, 12 July 2020 (UTC)
@Double sharp: This is primarily a type [6](d) argument i.e. omnibus fogging: unjustified reframing i.e. taking or reinterpreting my arguments out of context.
The age of a reference does not necessarily mean it becomes dubious. One exception I'd make is in the field of superconductivity.
Parish's explanation, on electrostatic grounds, is plausible and shows it isn't necessary to invoke f-orbital participation.
MMH are a mixed bag. They refer to either "possible" bonding from the f orbitals, or bond orders less than unity—which is it then? They then say "certain shapes (such as a regular cube) or lower coordination number also demand use or f-orbitals on symmetry grounds." Then they muck things up by following with, "but their occurrence in lanthanide or actinide element complexes 'suggest' the 'possibility' of f orbital participation. If the use of f orbitals are "demanded", why do they then say that this is [only] a "suggested" "possibility"?
I place more credibly in Parish. Sandbh (talk) 00:57, 13 July 2020 (UTC)
Update: Shchukarev (1974, pp. 118–119)
In our 2017 IUPAC submission we included an extract from: Shchukarev SA 1974, Neorganicheskaya khimiya, vol. 2, Vysshaya Shkola, Moscow (in Russian). This extract included a confusing sentence, as follows: "The exceptional uniqueness of Gd and Cm, akin to that of Mg and Ca [sic; italics added], would also be unclear." Courtesy R8R, I've looked at what Shchukarev wrote and can now correct the record.
Here's the relevant part of the IUPAC submission, and the corrected extract:
- Shchukarev (1974, p. 118) appears to support La-Ac on the grounds that the 4f shell does not start filling until Ce and that (effectively) the filling sequence—which runs from Ce to Lu—is periodic, with two periods. Thus, after the occurrence of a half-full 4f shell at Eu and Gd, the filling sequence repeats with the occurrence of a full shell at Yb and Lu (Rokhlin 2003, pp. 4–5). A similar, but weaker, periodicity (Wiberg 2001, p. 1643–1645) is seen in the actinides, with a half full 5f shell at Am (in the gas phase) and Cm, and a full shell at No and Lr. Placing Lu and Lr under Y obscures the start of the filling of the f block (it would appear to start at La) and visually truncates its double periodicity (it would be cut off at Yb whereas it would actually end in the d block).
- A translation of what Shchukarev wrote reads as follows:
- "If we […] considered the latter [Lu and 103] not as 4f and 5f elements but rather as members of 5d and 6d series, the d-electron prevention determining filling f vacancies as stable would be lost as well as the correctness of placing of imitators before Gd and Cm as well as Lu and 103. The exceptional uniqueness of Gd and Cm, akin to that of Mn and Zn, would also be unclear."
- "Analysis: We agree with the basis of Shchukarev's support for -La-Ac, noting the most important periodic property of the Ln and An is their valency. Thus, in the Ln, we see the analogous +2 ions of Eu and Yb, and the +4 ions of Ce and Tb (Wiberg 2001, p. 1644–1645). The double periodicity of the Ln and An is further explored by Ternstrom (1976) and (for the Ln only) by Horovitz and Sârbu (2005, pp. 473, 483). The former treats the Ln as running from Ce–Lu; the latter refers to 15 Ln from La to Lu. For reasons previously explained we think the approach of Horovitz and Sârbu lacks rigour."
Shchukarev's interpretation thus hinges on three considerations:
(a) the delayed start of filling of the 4f subshell; (b) the f7 imitators Eu and Am before f7 Gd and Cm, and the f14 imitators Yb and No before f14 Lu and Lr; and (c) the analogies between Gd and Cm, and Mn and Zn.
This double periodicity had been noted by Klemm in 1929 on the basis of atomic structure (Remy 1956, p. 492); and by Sobolev (2000, pp. 44–45).
Shchukarev has some other interesting figures in his book; it will take me a while to work out what they mean.
It's good to now be able to correct the record. Sandbh (talk) 01:37, 10 July 2020 (UTC)
References
- Horovitz O & Sârbu C 2005, "Characterisation and classification of lanthanides by multivariate-analysis methods", Journal of Chemical Education, vol. 82 no. 3, pp. 473–483, doi: 10.1021/ed082p473
- Remy H 1956, Treatise on inorganic chemistry, vol 2, Elsevier, Amsterdam
- Rokhlin LL 2003, Magnesium alloys containing rare earth metals: Structure and properties, Taylor & Francis, London
- Sobolev BP 2000, The rare earth trifluorides: The high temperature chemistry of the rare earth trifluorides, vol. 1, Institut d'Estudis Catalans, Barcelona, pp. 44–45
- Ternström T 1976, "Subclassification of lanthanides and actinides", Journal of Chemical Education, vol. 53, no. 10, pp. 629–631
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego
- @Sandbh: (a) doesn't exist in any chemically or physically meaningful sense; (b) has it backwards (Gd and Cm are imitating the half-filled shells of Eu and Am, not the other way round; Lu and Lr are no more imitating Yb and No than Hf and Rf are); and we know that because (c) there are no analogies of Gd/Cm and Lu/Lr to Mn and Zn; graphing any relevant property makes it clear that it is Eu/Am and Yb/No that take those analogous positions. There's nothing in this that I and Droog Andrey haven't addressed and refuted already. Double sharp (talk) 01:51, 10 July 2020 (UTC)
@Double sharp:@Droog Andrey: I'll stick with Schukarev given his life, achievements, academic record, and reputation. Sandbh (talk) 03:36, 10 July 2020 (UTC)
- @Sandbh: And I'll stick with Landau and Lifschitz. Two can play this game.
- And I notice that once it is not about his support for La, you stop sticking with Shchukarev, bearing in mind that he is the source of the term "kainosymmetry" that you criticised me for putting into the periodic table article above. Double sharp (talk) 04:22, 10 July 2020 (UTC)
@Double sharp: I've already noted the shortcomings of L&L. I'll look again at S on kainosymmetry. Sandbh (talk) 05:02, 10 July 2020 (UTC)
- So, as we can see, credentials only end arguments when they are held by authors who agree with Sandbh. When held by authors that don't agree with Sandbh, he can find "shortcomings". Double sharp (talk) 05:44, 10 July 2020 (UTC)
@Double sharp: Yes, that was the approach you and I took in our IUPAC submission. Sandbh (talk) 06:17, 10 July 2020 (UTC)
- @Sandbh: Nope. We analysed all arguments. Badly and incompletely as I now see in hindsight, since I know more now, but we didn't invoke the credentials of the authors selectively. Double sharp (talk) 06:31, 10 July 2020 (UTC)
@Double sharp: For authors who supported La we mostly agreed with them. For authors who supported Lu we went to extra lengths to discredit their arguments. That is what I call selective. And please leave me out of your aspersion that "we" analysed all arguments badly and incompletely. There is plenty of good content in that paper. Sandbh (talk) 07:44, 10 July 2020 (UTC)
- @Sandbh: Nope. Your analyses, not just my old ones, are part of what I and Droog Andrey have been criticising. He since 2018, me since 2019. And we didn't decide in advance to mostly agree with La arguments and go to extra lengths to discredit them. That would be selective. We pointed out flaws with some La arguments too. Double sharp (talk) 08:17, 10 July 2020 (UTC)
The following text in that submission, in which a La argument is criticised, is apparently a hallucination:
| “ | On basic character he says that increasing basicity with increasing atomic number is a general principle for the entire periodic system and since Sc-Y-La follows this pattern, whereas Sc-Y-Lu does not, he overall favours La in group 3.
... We think this argument is inconclusive as, while increasing basicity is seen as general principle across the periodic table there are exceptions (group 12, for example) and Trinfonov does not address the question of whether this would be the case for group 3. |
” |
Double sharp (talk) 08:24, 10 July 2020 (UTC)
- Like I said, “For authors who supported La we mostly agreed with them.” “Most” means “not all” as per this example. Sandbh (talk) 10:19, 11 July 2020 (UTC)
- But we didn't do it just because they agreed with La. We held the La arguments to the same standards as the Lu ones; it is simply that, because we had an incomplete understanding of the situation, it resulted in the Lu arguments appearing like they mostly did not hold water. (Cf. all the "let's look at group 2" rebuttals of Lu. They don't really work because the blocks come first.) That's not what you're doing now. You apply a double standard in how you treat La arguments vs how you treat Lu arguments. That precisely matches what I said: 'So, as we can see, credentials only end arguments when they are held by authors who agree with Sandbh. When held by authors that don't agree with Sandbh, he can find "shortcomings".' Double sharp (talk) 10:27, 11 July 2020 (UTC)
From Archive 44:
| “ | Sandbh I found this comment rather interesting and this made me question one certain thing. You said you were looking for observations that collectively support -La-Ac. Do you think that this is a good objective in the first place? My normal understanding is that one should look for observations and then make a judgment: make observations, assess them, see what different combinations of them produce, and then, having done that, cast a judgment. I realize that your modus operandi sounds like you have a hypothesis, for which argumentation in support is sought; but how critical are you on it and what about argumentation against the hypothesis? Regardless, if my understanding I've just described is correct, then the hypothesis MO does not appear to be the right one, because this is not really an observation problem; this is a classification problem.
I have noted in the very beginning of this discussion, which started with an article you wrote, that it did not appear to me that pro-La-Ac and pro-Lu-Lr arguments were given the same weight, and I said, perhaps not as explicitly but to the same meaning, that it looked like this was done so deliberately so that one option is favored over the other. I am afraid that what I've read so far reinforces this thinking within me. Please correct me if my thinking here is wrong.--R8R (talk) 16:37, 26 April 2020 (UTC) ... I was not rushing to accuse Sandbh of being unfair because I, while disagreeing, thought that this thinking is honest. I'm not saying this because that would make me look polite or because I'm afraid of confrontation: I mean what I said, even when I happen to disagree not just with the outcome, but also with the thinking itself. And I said before, Sandbh is trying his best, and one can only do so much in science. In that respect, I think similarly of Double sharp's thinking. I also think that not only I can disagree with someone, but I also find it important to remember that others can disagree with me, and their ideas are valuable in that case, too. However, when I said that it looked like the two options were not judged equally, I got an upfront "that's right." I was expecting to hear something like, "I tried and this is just what it looks like," something that does not resemble what I said. I can only resort to that the quoted piece of introduction is up for interpretation. I am most certain Double sharp could write something like that but for -Lu-Lr. "Vernon (2020) notes that Ce3+ through Lu3+ have 1 through 14 f electrons, which is a good resemblance of a block. However, you look at atoms rather than ions elsewhere in the periodic table to define a block, and it remains unclear why this case should be an exception." And no agreement could ever be reached unless one side resigns from discussion, or unless the tactic is changed. To resolve an argument, I'd propose first determining the criterion which allows us to say something is right or not. And if a solution is truly sought, I'd heartily propose establishing that common sense of criteria before applying it to both options. (Maybe that's been tried, please tell me if it has, this discussion is getting so out of hand with its size I couldn't possibly tell.) What's the difference between an article that is set to put -La-Ac over -Lu-Lr and an article that is set to put phlongiston over oxygen burning? We wouldn't write a latter one today because we know that an equal approach would debunk the phlongiston concept. Is -La-Ac strong enough to stand a similar test from -Lu-Lr?--R8R (talk) 20:16, 29 April 2020 (UTC) |
” |
How exactly one can "say something is right or not", when one of the participants (Sandbh) has previously said "Logic doesn't apply in this case, only one's strength of arguments" (never mind how to measure that without logic), and continues to deny the relevance of classical logic (including reductio ad absurdum) to the problem, is left as an exercise to the reader.
Meanwhile, let us see what everyone else thinks:
- "La arguments are totally local, while Lu arguments are pretty regular. That exactly matches Ptolemy vs. Copernicus. The history just repeats itself. Nothing more to say." - Droog Andrey
- "Cool, thanks. This makes perfect sense. Well said. Team Lu for me!" - Dreigorich
- "I'd really just want to get rid of the Scandium/Yttrium overhang in the long periodic table. It is ugly and very artificial IMO." - Officer781
- "I am more convinced by the Lu arguments I've read, and given the support of the scientific community and lack of consensus against it here (no "battleship" as you describe), I'll join the RfC once it's underway." - ComplexRational
- "Even worse, a publication may be biased towards a preference for more simple or elegant or 'by authority' (IUPAC) form, which is a beauty contest not science. I understand Sandbh does argue for such criteria seriously, but that still does not convince me—FWIW." - DePiep
And of course, I will quote Jensen instead of saying something new:
| “ | When it comes to the question of why La and Ac should remain in the d-block rather than being reassigned to the f-block, Lavelle offers no new chemical or physical evidence other than his constant reiteration of the fact that both elements contain d-electrons in their ground-state valence configurations, but no f-electrons. Yet in the cases of both Lu and Th, for which this is equally true, he proceeds to inconsistently argue that this fact is of no consequence when it comes to assigning them to the f-block. As with the case of the revised configuration for Lr, which counts when it comes to not placing this element in the d-block but is irrelevant when it comes to placing it in the f-block, this arbitrary and naive use of electron configurations, to the exclusion of all other evidence, is logically inconsistent and leaves one with the impression that the only true argument that Lavelle has for the major premise of his diatribe is that La and Ac should remain in the d-block because that is where IUPAC places them in its official periodic table [which it doesn't, so you'd have to substitute "Authorities So-and-So who actually don't analyse the situation"] and therefore all rational discussion of other possibilities is strictly forbidden. | ” |
Double sharp (talk) 12:53, 11 July 2020 (UTC)
Revealing text
A rather revealing bit from this new archive 46 appears as follows:
| “ | I've switched back and forth between La and Lu. If IUPAC comes out for Lu that'd be fine by me. Would I've been wrong? No, I would've made a mistake, and would look forward to new learning. "Wrong" is negative, emotive, pejorative, baggage-like, and unhelpful. ... Sandbh (talk) 06:08, 10 May 2020 (UTC) | ” |
It appears, however, that everybody else on the talk page coming out for Lu is not enough for Sandbh to suspect that he just might be wrong. Judging from Archive 42, it appears that this is a manifestation of the phenomenon that Sandbh in this thread only seems to be open to learning from sources he likes enough to quote. And even then it seems rather selective, given that in this case not even Greenwood and Earnshaw, which he himself has referred to elsewhere here when it seems to support his favoured classification of the Ln as Ce-Lu only (never mind how often La too appears in that chapter) was enough.
| “ | There’s nothing particularly weird about group 3 acting more like groups 1 and 2. [Sandbh (talk) 00:06, 25 January 2020 (UTC)]
Indeed, since groups 4 and 5 also act more like groups 1 and 2 very often, so the argument does not amount to that much in hindsight. Double sharp (talk) 00:20, 25 January 2020 (UTC) @Double sharp: That’s complete nonsense. I’m astonished to think you could post such rubbish. For the seven thousandth time, as per the literature, groups 1 to 3 have a predominately ionic chemistry. Groups 4 and 5 have a predominately covalent chemistry. End of story. The end. Period. Sandbh (talk) 06:48, 26 January 2020 (UTC) Look, there is no such thing as a complete volte-face from ionic to covalent. It depends on what the counter-anion is. We go from ionic to metallic (which you're overlooking completely) across the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na. And that's in group 1, with an example taken straight from Greenwood & Earnshaw p. 81. Ionic vs. covalent is (1) gradual, from covalent to polar covalent to mildly ionic to strongly ionic, depending on the difference of electronegativity between the two elements; (2) not a complete dichotomy, because you overlook metallic bonding; and (3) not split by elements, but rather by electronegativity differences, which have a lot to do with oxidation state (just compare uranium chemistry as we jack the oxidation state up from +3 to +4 to +5 to +6). So I'm astonished to see you put so much weight on "ionic vs. covalent" as a false dichotomy. (And as I keep saying, the general thing across the periodic table is continuity, and the sharp dichotomies you like to point to have a distinct tendency to not exist.) The whole literature, when it sees fit to split "main group" from "transition", universally uses other criteria like "variable oxidation states", "coloured compounds from d–d transitions", "a wide variety of complexes", "formation of paramagnetic compounds". Ionic vs. covalent has nothing to do with it, and nobody uses that as a criterion in the literature for this divide. Respectfully, I put it to you that Zr, Hf, Nb, and Ta by this standards have weak credentials as transition metals, just as weak as Sc for that matter. Double sharp (talk) 10:32, 26 January 2020 (UTC) [JUST OVER TWO MONTHS OF FUTILE ARGUING LATER]
@Double sharp: We’ve been discussing whether or not this exists. We’ve argued about predominately ionic v predominately covalent. You’ll recall the extract from Rayner-Canham and Overton: "For chemists…the most important feature of an element is its pattern of chemical behaviour, in particular, its tendency toward covalent bond formation (or its preference for cation formation)." (p. 29) They add some nuance: "The Ionic-Covalent Boundary Unfortunately, there is no firm predictable boundary between ionic and covalent behaviour for solid compounds of metals and nonmetals. As predicted from Fajans’ first rule, increasing theoretical cation charge results in increasing charge density, which will favour covalent behaviour. However, as predicted by Fajans’ second rule, the anion also plays a role: thus as the metal oxidation state increases, the iodide is first likely to exhibit a low melting point, then the bromide, then the chloride, and finally the fluoride and oxide." (p. 99) "…inorganic chemists see not a rigid ionic-covalent divide but a bonding continuum. Figure 5.11 shows electron density profiles for four points on this continuum: the pure covalent, a polar covalent bond, a polarised ionic bond, and a pure ionic bond. The ratio of ionic to covalent character can be defined as the difference in electronegativities…between the pairs of atoms. Thus, pairs of atoms with…[a difference] close to zero will possess essentially pure covalent bonds with equally shared electrons, whereas those…> 3.0 are regarded as purely ionic… (p. 109)" They go on to refer to the Van Arkel-Ketelaar bond triangle with its "rough" division into metallic, ionic, and covalent “zones” and Laing’s extension of the triangle into a tetrahedron. These kinds of divisions, while rough, are nevertheless valuable. As expressed by Nelson (2011): "…care needs to be taken to remember that…[this classification scheme] is only an approximation, and can only be used as a rough guide to the properties of the elements. Provided that this is done, however, it constitutes a very useful classification, and although purists often despise it because of its approximate nature, the fact is that practising chemists make a great deal of use of it, if only subconsciously, in thinking of the chemistry of different elements. [Sandbh (talk) 09:46, 1 April 2020 (UTC)] @Sandbh: Using those four categories is so much better than "predominantly ionic" or "predominantly covalent", so I'm glad we have come to some agreement on this. They are in fact the reason why I find "predominantly ionic" and "predominantly covalent" not useful: using those terms sweeps under the rug the very trend that is so clearly controlling it, that of electronegativity difference as Rayner-Canham and Overton are saying. Double sharp (talk) 13:09, 1 April 2020 (UTC) @Double sharp: Quite so. Sandbh (talk) 01:08, 2 April 2020 (UTC) @Sandbh: Well, I have been saying exactly that since January: “Look, there is no such thing as a complete volte-face from ionic to covalent. It depends on what the counter-anion is. We go from ionic to metallic (which you're overlooking completely) across the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na. And that's in group 1, with an example taken straight from Greenwood & Earnshaw p. 81. Ionic vs. covalent is (1) gradual, from covalent to polar covalent to mildly ionic to strongly ionic, depending on the difference of electronegativity between the two elements; (2) not a complete dichotomy, because you overlook metallic bonding; and (3) not split by elements, but rather by electronegativity differences, which have a lot to do with oxidation state (just compare uranium chemistry as we jack the oxidation state up from +3 to +4 to +5 to +6). So I'm astonished to see you put so much weight on "ionic vs. covalent" as a false dichotomy. (And as I keep saying, the general thing across the periodic table is continuity, and the sharp dichotomies you like to point to have a distinct tendency to not exist.)” But it looks like it's only admissible when Rayner-Canham and Overton say it, not when I say it, even if it is absolutely normal high school chemistry material (which I learnt too). Now, the same thing is going on for the supposed volte-face between group 3 and group 4 on the basis of aqueous chemistry: it doesn't exist, all you have is oxidation states, atomic radius, and electronegativity too, exactly like I remember from high school chemistry. Or do I have to quote Wulfsberg explicitly? ;) Here's a quote from p. 94 of his Principles Of Descriptive Inorganic Chemistry: “Because the acidity of a cation rises rapidly with its charge, there are several d-block elements possessing several oxidation states (such as chromium) that have one or more oxides that show only basic properties (e.g. chromium(II) oxide, CrO), one or more oxides that are amphoteric (e.g. chromium(III) oxide, Cr2O3), and one or more oxides that possess only acidic properties (e.g. chromium(VI) oxide, CrO3). Clearly, the higher the oxidation number of a given element, the more acidic the corresponding oxide will be.” Zero mention of group divides in his whole section on periodic trends in acid-base and solubility properties of oxides. Which is because there is no such thing. Double sharp (talk) 14:04, 2 April 2020 (UTC) |
” |
^_^ Double sharp (talk) 14:14, 11 July 2020 (UTC)
Revealing text
A rather revealing bit from this new archive 46 appears as follows:
| “ | I've switched back and forth between La and Lu. If IUPAC comes out for Lu that'd be fine by me. Would I've been wrong? No, I would've made a mistake, and would look forward to new learning. "Wrong" is negative, emotive, pejorative, baggage-like, and unhelpful. ... Sandbh (talk) 06:08, 10 May 2020 (UTC) | ” |
It appears, however, that everybody else on the talk page coming out for Lu is not enough for Sandbh to suspect that he just might be wrong. Judging from Archive 42, it appears that this is a manifestation of the phenomenon that Sandbh in this thread only seems to be open to learning from sources he likes enough to quote. And even then it seems rather selective, given that in this case not even Greenwood and Earnshaw, which he himself has referred to elsewhere here when it seems to support his favoured classification of the Ln as Ce-Lu only (never mind how often La too appears in that chapter) was enough.
| “ | There’s nothing particularly weird about group 3 acting more like groups 1 and 2. [Sandbh (talk) 00:06, 25 January 2020 (UTC)]
Indeed, since groups 4 and 5 also act more like groups 1 and 2 very often, so the argument does not amount to that much in hindsight. Double sharp (talk) 00:20, 25 January 2020 (UTC) @Double sharp: That’s complete nonsense. I’m astonished to think you could post such rubbish. For the seven thousandth time, as per the literature, groups 1 to 3 have a predominately ionic chemistry. Groups 4 and 5 have a predominately covalent chemistry. End of story. The end. Period. Sandbh (talk) 06:48, 26 January 2020 (UTC) Look, there is no such thing as a complete volte-face from ionic to covalent. It depends on what the counter-anion is. We go from ionic to metallic (which you're overlooking completely) across the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na. And that's in group 1, with an example taken straight from Greenwood & Earnshaw p. 81. Ionic vs. covalent is (1) gradual, from covalent to polar covalent to mildly ionic to strongly ionic, depending on the difference of electronegativity between the two elements; (2) not a complete dichotomy, because you overlook metallic bonding; and (3) not split by elements, but rather by electronegativity differences, which have a lot to do with oxidation state (just compare uranium chemistry as we jack the oxidation state up from +3 to +4 to +5 to +6). So I'm astonished to see you put so much weight on "ionic vs. covalent" as a false dichotomy. (And as I keep saying, the general thing across the periodic table is continuity, and the sharp dichotomies you like to point to have a distinct tendency to not exist.) The whole literature, when it sees fit to split "main group" from "transition", universally uses other criteria like "variable oxidation states", "coloured compounds from d–d transitions", "a wide variety of complexes", "formation of paramagnetic compounds". Ionic vs. covalent has nothing to do with it, and nobody uses that as a criterion in the literature for this divide. Respectfully, I put it to you that Zr, Hf, Nb, and Ta by this standards have weak credentials as transition metals, just as weak as Sc for that matter. Double sharp (talk) 10:32, 26 January 2020 (UTC) [JUST OVER TWO MONTHS OF FUTILE ARGUING LATER]
@Double sharp: We’ve been discussing whether or not this exists. We’ve argued about predominately ionic v predominately covalent. You’ll recall the extract from Rayner-Canham and Overton: "For chemists…the most important feature of an element is its pattern of chemical behaviour, in particular, its tendency toward covalent bond formation (or its preference for cation formation)." (p. 29) They add some nuance: "The Ionic-Covalent Boundary Unfortunately, there is no firm predictable boundary between ionic and covalent behaviour for solid compounds of metals and nonmetals. As predicted from Fajans’ first rule, increasing theoretical cation charge results in increasing charge density, which will favour covalent behaviour. However, as predicted by Fajans’ second rule, the anion also plays a role: thus as the metal oxidation state increases, the iodide is first likely to exhibit a low melting point, then the bromide, then the chloride, and finally the fluoride and oxide." (p. 99) "…inorganic chemists see not a rigid ionic-covalent divide but a bonding continuum. Figure 5.11 shows electron density profiles for four points on this continuum: the pure covalent, a polar covalent bond, a polarised ionic bond, and a pure ionic bond. The ratio of ionic to covalent character can be defined as the difference in electronegativities…between the pairs of atoms. Thus, pairs of atoms with…[a difference] close to zero will possess essentially pure covalent bonds with equally shared electrons, whereas those…> 3.0 are regarded as purely ionic… (p. 109)" They go on to refer to the Van Arkel-Ketelaar bond triangle with its "rough" division into metallic, ionic, and covalent “zones” and Laing’s extension of the triangle into a tetrahedron. These kinds of divisions, while rough, are nevertheless valuable. As expressed by Nelson (2011): "…care needs to be taken to remember that…[this classification scheme] is only an approximation, and can only be used as a rough guide to the properties of the elements. Provided that this is done, however, it constitutes a very useful classification, and although purists often despise it because of its approximate nature, the fact is that practising chemists make a great deal of use of it, if only subconsciously, in thinking of the chemistry of different elements. [Sandbh (talk) 09:46, 1 April 2020 (UTC)] @Sandbh: Using those four categories is so much better than "predominantly ionic" or "predominantly covalent", so I'm glad we have come to some agreement on this. They are in fact the reason why I find "predominantly ionic" and "predominantly covalent" not useful: using those terms sweeps under the rug the very trend that is so clearly controlling it, that of electronegativity difference as Rayner-Canham and Overton are saying. Double sharp (talk) 13:09, 1 April 2020 (UTC) @Double sharp: Quite so. Sandbh (talk) 01:08, 2 April 2020 (UTC) @Sandbh: Well, I have been saying exactly that since January: “Look, there is no such thing as a complete volte-face from ionic to covalent. It depends on what the counter-anion is. We go from ionic to metallic (which you're overlooking completely) across the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na. And that's in group 1, with an example taken straight from Greenwood & Earnshaw p. 81. Ionic vs. covalent is (1) gradual, from covalent to polar covalent to mildly ionic to strongly ionic, depending on the difference of electronegativity between the two elements; (2) not a complete dichotomy, because you overlook metallic bonding; and (3) not split by elements, but rather by electronegativity differences, which have a lot to do with oxidation state (just compare uranium chemistry as we jack the oxidation state up from +3 to +4 to +5 to +6). So I'm astonished to see you put so much weight on "ionic vs. covalent" as a false dichotomy. (And as I keep saying, the general thing across the periodic table is continuity, and the sharp dichotomies you like to point to have a distinct tendency to not exist.)” But it looks like it's only admissible when Rayner-Canham and Overton say it, not when I say it, even if it is absolutely normal high school chemistry material (which I learnt too). Now, the same thing is going on for the supposed volte-face between group 3 and group 4 on the basis of aqueous chemistry: it doesn't exist, all you have is oxidation states, atomic radius, and electronegativity too, exactly like I remember from high school chemistry. Or do I have to quote Wulfsberg explicitly? ;) Here's a quote from p. 94 of his Principles Of Descriptive Inorganic Chemistry: “Because the acidity of a cation rises rapidly with its charge, there are several d-block elements possessing several oxidation states (such as chromium) that have one or more oxides that show only basic properties (e.g. chromium(II) oxide, CrO), one or more oxides that are amphoteric (e.g. chromium(III) oxide, Cr2O3), and one or more oxides that possess only acidic properties (e.g. chromium(VI) oxide, CrO3). Clearly, the higher the oxidation number of a given element, the more acidic the corresponding oxide will be.” Zero mention of group divides in his whole section on periodic trends in acid-base and solubility properties of oxides. Which is because there is no such thing. Double sharp (talk) 14:04, 2 April 2020 (UTC) |
” |
^_^ Double sharp (talk) 14:14, 11 July 2020 (UTC)
Typology of arguments
@Дрейгорич, R8R, Droog Andrey, ComplexRational, Officer781, and DePiep:
I'm summoning you here:
- in memory of Double sharp's summons to an exchange with me that he thought was so hilarious; and
- in response to his view that the fact that no one else supports me means something.
Happy reading for those of you who do so.
@Double sharp: Before I start, there are some things we agree on. For example, we agree the trend of ionic radii going down group 3 is smoother for Sc-Y-La-Ac. Equally, we agree the trend of melting points going down group 3 looks smoother for Sc-Y-Lu-Lr.
Now, in looking back through the mega-thread, I can discern seven types of argument you employ:
- They must be wrong, of course
- A zombie that will not die
- Drill-down obfuscation
- Conflationary fallacy
- Obfuscation by irrelevant extension
- Omnibus fogging
- No one else supports me.
[1] They must be wrong, of course
I don’t need to say much here apart from listing a range of authors who, according to you, are wrong:
|
Bent |
Einstein |
Mingos |
Scerri |
Sobolev |
- *(1976), with 50,522 citations, all of whom are unable to compare like-with-like, and not realising that Shannon's measurements must be noise compared to the differences in high spin low spin ions
- And also never mind that Shannon himself gives separate numbers for high spin low spin ions ^_^ Double sharp (talk) 07:29, 12 July 2020 (UTC)
[2] A zombie that will not die
This type refers to an argument that I have shown has no basis, yet you keep bringing it back. The best example is that Th has no f electron in the gas phase and that this therefore represents a fatal inconsistency in arguing for the start of the f-block at Ce.
As Jones has written:
- "Scientists should not lose sleep over the hard cases. [italics added] As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics." Jones 2010, Pluto: Sentinel of the outer solar system, Oxford University Press, p. 171).
As Schwerdtfeger, Smits & Pyykkö (2020) write:
- "Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place [italics added] in the PTE, keeping their distinctive quantum nature in mind."
- "Fuzzy concepts like chemical similarity often lead to unnecessary [italics added] disputes concerning the PTE."
Another example is the well worn symmetry saw, "Meanwhile, it's self-evidently obvious that a Lu table is more symmetric and regular than a La table."
This is a zombie argument. There is no basis to regard regularity or symmetry as fundamental requirements (Scerri 2004, p. 149; 2019, p. 385). As Eugen Schwarz (2019, pers. comm., 8 Dec) stated, "The real, rich pattern of elements' chemistry does not fit into a clear-cut rectangular grid."
[3] Drill-down obfuscation
This refers to my arguments based on an 80/20 principle, or the broad contours of the situation. You then drill down into the second-order 20 per cent of dips, folds, and cracks and argue that therefore my first-order generalisation is invalid.
A nice example is the dualistic behaviour of the d10s1 group 11 metals, Cu, Ag, and Au. They can act as main group metals or transition metals. Their dualistic behaviour is a byproduct of their anomalous configurations or differentiating electrons i.e. d10s1 as opposed to their expected configurations of d9.
For example, Steele 1966, The chemistry of the metallic elements, p. 67:
- "The overlap in properties between the b-subgroup metals and the transition metals is shown in the properties of copper, silver, and gold. In their monovalent compounds they are typical b-subgroup elements. The d-electrons can, however, be used in bond formation to give compounds of the elements in the divalent and trivalent states. In these compounds the d-subshell is incomplete and their chemistry is typical of transition metal compounds."
To discredit my argument you drill down, as follows:
- "The differing behaviours of group 11 metals are simply explained from the relative energy differences between (n-1)d and ns (the chemically active valence sub-shells) at the end of the d-block. In Cu the gap is not so big, we see +1 and +2 states frequently. In Ag it is bigger, in Au it is smaller again because of relativistic destabilisation of 5d. That's what controls the ionisation energies and hence common oxidation states, not the differentiating electrons."
The level of detail you enter into is not needed. I agree with Scerri who believes there is great merit in taking as philosophical, and as abstract as possible, an approach to the periodic table. It's not necessary to drill down.
Friedrich, a physicist, has written on this:
- "Exact sciences cherish approximations. [italics added] More often than not, resorting to approximations is a matter of necessity…that is the case when a problem cannot in principle be solved exactly. For instance, many-body problems fall all in this category, whether they are classical or quantum…We note that here many means more than two; hence there are very many many-body problems. Approximations are also introduced when seeking a qualitative understanding of a problem: approximations (called in this context models or treatments) reveal the structure of problems and aid in identifying analogies with other problems, thus adding to the sense that we can make of them…
- "Approximations to the solution of a certain problem form a hierarchy, according to the degree of their accuracy; for many problems, an arbitrary accuracy can be achieved in principle and often in practice. A good example is the N-electron atom problem."
[4] Conflationary fallacy
By this I mean taking an argument of a fixed scope, conflating it with a larger scope, and then arguing that since the conflated argument does not hold, neither therefore does the smaller scope argument.
A good example would be my observation about isodiagonal relationships. There are about 30 elements which show such relationships. Per Rayner-Canham, "Isodiagonality is, in some ways, a general attribute of the properties of the chemical elements. For example, the metal-nonmetal divide forms an almost diagonal demarkation (Edwards and Sienko 1983). Similarly, the elements often considered to be semimetals fall on a roughly diagonal border between the metals and nonmetals (Hawkes 2001)."
Your response? Such relationships are not generalisable to the whole periodic table therefore they don't mean anything. Thus, most of the elements don't display the properties of the noble gases. Therefore the NG don't mean anything.
[5] Obfuscation by irrelevant extension
A nice example of this is where you said:
- "Suppose, counterfactually, that lanthanum really were [Xe]4f16s2 in the ground state. Then I doubt you would be arguing for the f block to start at Ce. Actually I doubt anyone would be arguing that. But alas! That f electron would still go missing in the +3 cation, and the argument would still support Ce-Lu!"
So, the basis of your assertion rests on (1) an out-of-universe hypothetical situation; (2) applicability to other blocks in which there are no delayed starts to filling the applicable sub-shells.
On this irrelevant basis, I must be wrong.
Either that or I am accused of:
- "…more refusal to accept the principle of reductio ad absurdum, universally accepted by anyone who wants his or her science to be based on standard, classical, logic."
Once again, we have obfuscation by irrelevant extension i.e. expecting classical (black and white) logic to apply to fuzzy logic. See also Jones (2010), and Schwerdtfeger, Smits & Pyykkö (2020).
[6] Omnibus fogging
This is a left-over type of argument, encompassing but not limited to: (a) catastrophizing e.g. if you do that, logic demands Be-Mg will have to be moved into group 12[!]; (b) tangentialising i.e. making an irrelevant observation such as focussing on the physical aspects of double periodicity among the Ln, never mind the chemical aspects; (c) word-count bombardment i.e. counter-responses to my evidence are generally five times as long;* or (d) unjustified reframing i.e. taking or reinterpreting my arguments out of context. I include here the bizarre and unreasonable interpretation^ that a periodic table of the form Sc-Y-La*-Ac** with a 14-element wide f-block, can be construed as actually referring to a 15-element wide f-block.
- *This is a classic sign of not having a strong response and needing to resort to multiple weak arguments and minutiae in order to shore up one's position
- ^An unreasonable interpretation is one that is so unreasonable that no reasonable person would make it.
[7] No one else supports me
And your science-based point is?
You criticise my lack of a scientific approach yet the lack of support you are referring to is that from within our small project, which amounts to no more than untested personal opinions. These personal opinions (including my own) mean nothing more than that, and nothing from the perspective of the literature.
Mendeleev didn't exactly have a rapturous welcoming committee.
In 1982, two Australian scientists, Robin Warren and Barry Marshall, identified Helicobacter pylori as causative factor for ulcers. Their paper was poorly received–yet went on to be widely accepted. Go figure.
You bagged my nonmetal categories and the magic thread linking the intermediate nonmetals. Subsequently my peer-reviewed article is published in Foundations of Chemistry, and records 1,910 accesses to date. Rayner-Canham will be citing it in some educational material to be published by the ACS.
I have support from: Atkins et al; Clarke and White; C&W; G&E; Lavelle, and "the silent majority"; Liu et al.; Myers, Oldham and Tocci; Remy; Restrepo; Rokhlin; Shchukarev; Sobolev; Ternström; the IUPAC project survey; three FoC peer reviewers; and Wiberg.
This type of argument, i.e. no one in our small project supports you, is neither here nor there.
Postscript
I've posted this typology here, for the record. Sandbh (talk) 06:36, 12 July 2020 (UTC)
- Except, of course, that the most important thing for getting a change through on WP is [7]. Double sharp (talk) 07:29, 12 July 2020 (UTC)
- Agree with Double sharp. ― Дрейгорич / Dreigorich Talk 07:32, 12 July 2020 (UTC)
- @Double sharp, Дрейгорич, and R8R: The most important thing for getting a change through on WP is consistency with the literature. For example:
- we changed group 12 from transition metals to post-transition metals since a literature survey of periodic tables was about 50/50 on this point;
- on the question of which elements are metalloids, we settled this on the basis of a survey of the literature; and
- for organising the nonmetals, the literature is a shambles, so any considered approach will do, as per what we did, after considerable discussion.
- @Double sharp, Дрейгорич, and R8R: The most important thing for getting a change through on WP is consistency with the literature. For example:
- For group 3, subject to the findings of the IUPAC project, and the reception accorded to it by the scientific community, there is no consensus for change. The literature is 4:1:1 towards Sc-Y-La-Ac. Jensen gave it a go in 1983, and there was a flurry of interest in Sc-Y-Lu-Lr, but it did not catch on. There have been some pro-Lu articles since that time, and there have been more of these than pro-La articles. I attribute that to Lavelle's silent majority; and to the lack of interest and excitement in compiling a pro-La paper, given the dominance of the La form. Sandbh (talk) 00:20, 13 July 2020 (UTC)
- That, of course, must be why we call At a metalloid and don't call Po one, even though 48% of metalloid lists include Po and only 40% include At. Double sharp (talk) 15:31, 14 July 2020 (UTC)
- For group 3, subject to the findings of the IUPAC project, and the reception accorded to it by the scientific community, there is no consensus for change. The literature is 4:1:1 towards Sc-Y-La-Ac. Jensen gave it a go in 1983, and there was a flurry of interest in Sc-Y-Lu-Lr, but it did not catch on. There have been some pro-Lu articles since that time, and there have been more of these than pro-La articles. I attribute that to Lavelle's silent majority; and to the lack of interest and excitement in compiling a pro-La paper, given the dominance of the La form. Sandbh (talk) 00:20, 13 July 2020 (UTC)
- Sorry for not writing anything despite all those pings I received. I'm in no shape to write much right now, but I'll say that the 4:1:1 consideration will be very important for the upcoming RfC, especially given that it's something that can be found on IUPAC's official website. I agree that consistency with literature is of the uttermost importance for Wikipedia if literature itself goes shows such a consistency; Wikipedia is meant to be a tertiary source. It'll be the thing that will hold me from supporting the motion on WP right now. 4:1:1 is nothing like 48% vs. 40% (I assume both percentages allow us to apply our own judgment).
- We'll see what things will be like if IUPAC settles on -Lu-Lr. Maybe there'll be a big change over time. Maybe there won't. I am personally displeased with how we apply their rule on spellings of elements (the way the Union itself does not apply it) and that makes me cautious to blindly follow whatever IUPAC suggests.--R8R (talk) 16:51, 19 July 2020 (UTC)
@DePiep: So, it looks like from point [6] that Sandbh thinks you are not a reasonable person! XD Double sharp (talk) 07:40, 12 July 2020 (UTC)
The Sandbh approach:
| “ | A mathematician is showing a new proof he came up with to a large group of peers. After he's gone through most of it, one of the mathematicians says, "Wait! That's not true. I have a counter-example!"
He replies, "That's okay. There's no need to lose sleep over the hard cases by drilling down. Also, I have two proofs." And a second mathematician shouts, "If your second step was true, then would be rational!" And he replies, " is not within the scope of my argument. You are making a conflationary fallacy." Yet a third mathematician shouts, "I have just completed a difficult four-page proof of the negation of your Lemma 3.2!" And he replies, "That's word-count bombardment, a classic sign of not having a strong response." A fourth mathematician shouts, "You have used an approximation that is only true for large values of n to make claims about arbitrarily small n!" And he replies, "Exact sciences cherish approximations! I have cited many, many sources. They all must be wrong, of course." And a fifth one shouts, "The results of this source were refuted in this other paper!" And a sixth one adds, "And this source you cite for a common belief actually disproves it!" And a seventh one tries to reason with him, saying, "Does it not make you pause and consider that nobody in this chamber is convinced?" Finally he shouts, "Logic doesn't apply to this! Mathematics requires a delicate mix of physical intuition, pragmatic cleverness, and practical know-how to figure out what the promising approaches are. Classical, black and white logic, including proof by contradiction, is not applicable!" At this the organisers eject him. As he is dragged out of the room, he continually repeats that Galois didn't exactly have a rapturous welcoming committee either. |
” |
| — Expanded from a famous mathematics joke | ||
Double sharp (talk) 07:47, 12 July 2020 (UTC)
- @Дрейгорич and Double sharp:
- I like this a lot for its creativity and improvisation.
- That said, it's a good example of a type 5 obfuscation by irrelevant extension argument. And it sure doesn't represent the Sandbh approach.
- The irrelevance is extending a joke based on mathematical logic and thinking to the Group 3 situation.
- The Group 3 question cannot be resolved or falsified by proof. All one can do is make observations about the options, and then weigh them up. One can certainly say some observations or arguments are inconsistent. But that is not the point of the PT. It's impossible to have a PT show all patterns or relationships of interest or to be universally consistent. You have to choose your viewpoint or properties and patterns of interest. That is why H and He are where they are and the literature says, "Scientists should not lose sleep over the hard cases" and "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE", and so on. It is useful to have arguments for moving H over B, C or F, and He over Be, or to have them both floating above the table, etc, since different perspectives can shed new insights. Nevertheless, and despite the distinctive and clearly separate nature of H and He, which needs to be kept in mind, "almost every chemist agrees that we can leave these elements in their current place in the PTE." Same goes for group 3 as Sc-Y-La-Ac, and Lavelle's silent majority.
- So, nice try, but no cyber-cigar. Sandbh (talk) 23:50, 12 July 2020 (UTC)
- No comment. ― Дрейгорич / Dreigorich Talk 03:32, 13 July 2020 (UTC)
The technology of Sc, Y and the REM (1963)
Source: Kleber EV and Love B 1963, The technology of scandium, yttrium and the rare earth metals, McMillan, New York, p. 16
Nothing especially novel here, only the smooth integration of the lanthanides into the PT:
- "The most common form periodic arrangement of the elements starts with an alkali metal as the first member of each row, and ends with a noble gas…
- The first two rows start with lithium and sodium, and progress by the addition of successive outer electrons to neon and argon respectively.
- The third row starts with potassium and is followed by calcium, but starting with scandium, the next element, an inner shell of electrons is filled to form a transition series, which is completed after eight electrons are added at the element nickel. The row then resumes adding outer electrons with the element copper, and is finally completed with the krypton noble gas structure. Similarly the fourth row starts with rubidium, is followed by strontium, and then yttrium starts a transition series which is completed after eight electrons at palladium. The row is eventually completed with a stable xenon structure. In both of the above transition series the inner shell is only one or two electrons deep, and so the addition of electrons still produces signifiant changes in chemical properties.
- The fifth row starts with cesium and is followed by barium and lanthanum. Lanthanum starts another transition series. The succeeding members of this series are hafnium, tantalum, tungsten, etc. Between lanthanum and hafnium, however, an inner transition sequence occurs. The filling of this inner shell (under several outer shells of electrons) constitutes the rare earth elements starting with cerium and concluding with lutetium."
No fuss, no bother. Sandbh (talk) 03:26, 13 July 2020 (UTC)
- The Sandbh approach: simply sweep things under the rug and make no fuss about them. That'll solve the problem. Right until it sneaks up behind you and has you for lunch, i.e. a raised hand at the back of the classroom asking about thorium. Double sharp (talk) 07:46, 13 July 2020 (UTC)
@Double sharp: That's a type 2 zombie that will not die argument. Sandbh (talk) 10:25, 13 July 2020 (UTC)
@Дрейгорич, Droog Andrey, R8R, ComplexRational, Officer781, and DePiep: Let's see who here is convinced by my argument that putting thorium in the f block with [Rn]5f06d27s2, and yet barring actinium with [Rn]5f06d17s2 from entry, points to an inconsistency in the idea that a block starts when its type of electron first appears. Double sharp (talk) 12:04, 13 July 2020 (UTC)
- As Double sharp said, Th and Ac both allow f electrons to bond in compounds. Surely that's enough to justify membership of the f block? Also, considering that we know the structure of the periodic table, if Th is excluded, would Ce, a definite f-block element, be excluded? ― Дрейгорич / Dreigorich Talk 22:06, 13 July 2020 (UTC)
Relocation into archive 46
Per the above. So the Group 3 mega-thread now occupies archive #'s 42, 44, and 46.
- Shall we number the auto-archiv into #47? So that #46 can be reserved for this group-3 only? -DePiep (talk) 19:20, 14 July 2020 (UTC)
- @DePiep: Yes, I think that would be a good idea. Double sharp (talk) 04:44, 15 July 2020 (UTC)
- Done, even more: #46-#47-#48 are reserved for Group-3+. Please do not start a discussion in an organising thread like this, Double sharp .-DePiep (talk) 21:46, 15 July 2020 (UTC)
- @DePiep: OK, moved that into a new section below. I don't think reserving archives 47 and 48 will be necessary, since the discussion has gotten so futile that I've decided to (finally) stop replying to Sandbh, and we have a consensus already. The next time this comes up should be in the RFC shortly. Double sharp (talk) 02:29, 16 July 2020 (UTC)
- Done, even more: #46-#47-#48 are reserved for Group-3+. Please do not start a discussion in an organising thread like this, Double sharp .-DePiep (talk) 21:46, 15 July 2020 (UTC)
- @DePiep: Yes, I think that would be a good idea. Double sharp (talk) 04:44, 15 July 2020 (UTC)
The principles involved
I may be wrong but I don't believe Double sharp ever said Th and Ac both allow f-electrons to bond in compounds. Rather, I believe he contended that their effectively empty 5f orbitals may show some occupation by the electrons of whatever element they are bonded to.
As to the start of a block, and for the record, my suggestion has always been that a block starts with the first appearance of the applicable electron. So the s-block starts at H, the p- at B, d- at Sc, f- at Ce. That is consistent, with no exceptions.
What happens in subsequent rows is irrelevant e.g. the fact that in an Lu table the 5d row starts with a 5p element (Lr). As I keep hammering on, the principles involved have been set out in the literature. Here they are again, in chronological order:
I - "Exact sciences cherish approximations. More often than not, resorting to approximations is a matter of necessity…that is the case when a problem cannot in principle be solved exactly. For instance, many-body problems fall all in this category, whether they are classical or quantum…We note that here many means more than two; hence there are very many many-body problems. Approximations are also introduced when seeking a qualitative understanding of a problem: approximations (called in this context models or treatments) reveal the structure of problems and aid in identifying analogies with other problems, thus adding to the sense that we can make of them…" (Friedrich 2004) --- same applies to Th
II - "Approximations to the solution of a certain problem form a hierarchy, according to the degree of their accuracy; for many problems, an arbitrary accuracy can be achieved in principle and often in practice. A good example is the N-electron atom problem." (ditto) --- same applies to Th
III - "Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics." (Jones 2010) --- same applies to the case of Th
IV - "If one seeks for the maximum chemical utility…[one] should opt for the more 'unruly' tables. If one seeks maximum elegance and orderliness above all…[one] should favor the more regular representations." (Imyanitov 2015 pp. 153–154) --- same applies to Th
V - "…for the purpose of selecting an optimal periodic table we prefer to consider block membership as a global property in which we focus on the predominant differentiating electron." (Scerri and Parsons 2018, p. 151) --- same applies to Th
VI - "The real, rich pattern of elements' chemistry does not fit into a clear-cut rectangular grid." (Eugen Schwarz 2019, pers. comm., 8 Dec) --- same applies to Th
VII - "Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind." (Schwerdtfeger, Smits & Pyykkö (2020) --- same applies to Th
VIII - "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE." (ditto) --- same applies to Th
IX - "The realization that classification in general, and the specification of natural kinds, is not a purely ontological question forces us to confront the fact that seeking an objectively optimal periodic table is rather futile. We should accept that a degree of convention must be used in selecting a periodic table that can be presented as perhaps the best possible table that combines objective factors as well as interest dependence." (Scerri 2020) --- same applies to Th
I have hope since I once supported Lu in group 3 and then changed my mind back to La. Double sharp once supported Lu, changed his mind to La, then changed his mind back to Lu. So neither of us are stick-in-the-muds. Sandbh (talk) 02:20, 14 July 2020 (UTC)
Jumping around
- Sandbh seems to think there's an effective difference between dative covalent bonding, when the ligand contributes both electrons in the pair, and the "first-year chemistry" style of covalent bonding as in HF where each atom contributes one electron. Never mind, of course, that electrons are effectively indistinguishable. So, apparently the 3d metals never use their 4s electrons at all.
- The point is that La, Ac, and Th in compounds all use their valence f orbitals as bonding ones in the same way despite their odd ground-state configurations. It's exactly the same sort of thing as palladium 5s or lawrencium 6d. The f occupancy can come from electron transfer from their own d and s subshells as in the metals, or it can come from ligand-to-metal donation, but the chemically important result is the same.
- So, thorium and actinium are being treated differently despite effectively the same usage of their 5f orbitals. There is a term for this: a double standard.
- I've mostly given up on Sandbh ever since he's disclaimed the use of logic. Not to mention since he started lecturing Droog Andrey (an actual computational chemist) about the applicability of logic to chemistry(!):
| “ | Not all arguments are logic. Good examples are politics, economics, philosophy, and ethics, and the many qualitative aspects of chemistry. I do wonder if the two of you understand that. It seems not. Sandbh (talk) 05:11, 14 May 2020 (UTC)
@Sandbh: Politics, economics, philosophy, and ethics are not natural science. Chemistry is. Droog Andrey (talk) 12:09, 16 May 2020 (UTC)' @Droog Andrey: Not all arguments in chemistry are based on logic. "In many respects, computational chemistry is still an art, and relies upon a delicate mix of physical intuition, pragmatic cleverness, and practical know-how." Sandbh (talk) 13:11, 16 May 2020 (UTC) |
” |
- (Again, that's also true of mathematics. Good luck telling the mathematicians that not everything in their subject is based on logic.) So this is mostly to see if everyone else who still believes in the applicability of logic (to wit: basically the entire scientific community) is convinced.
- As for regarding whether Sandbh is a stick-in-the-mud (see his last sentence where he says "neither of us are stick-in-the-muds"; that's not me trying to insult him) Double sharp (talk) 14:56, 14 July 2020 (UTC), there's a very interesting phenomenon in this thread. Which is that something that is totally standard in chemistry is rejected whenever I tell it to him, until he finds it in a source that he likes. Observe what happened about the nonsensical idea about "predominantly ionic" vs "predominantly covalent" elements that he was pushing near the beginning of this as a reason to force group 3 apart from group 4: my continued statement that this is nonsense were rejected for months when stated by me, even when I quoted Greenwood and Earnshaw backing it up, but finally accepted by him when he saw it in Rayner-Canham(!). We left the topic of aqueous cations (particularly the +4 cations of the group 4 metals) before getting to the stage when he finally finds it in sources he would find acceptable (which seems distinct from finding it in sources at all, which is easily done for aquated Zr4+).
- Now, apparently Sandbh would be ready to learn from IUPAC, but not us (never mind that Droog Andrey is, again, an actual computational chemist):
| “ | I've switched back and forth between La and Lu. If IUPAC comes out for Lu that'd be fine by me. Would I've been wrong? No, I would've made a mistake, and would look forward to new learning. "Wrong" is negative, emotive, pejorative, baggage-like, and unhelpful. ... Sandbh (talk) 06:08, 10 May 2020 (UTC) | ” |
- However I do wonder how Sandbh would react if IUPAC used the consistency arguments Droog Andrey and I have been advocating to support Lu in the table. Would he change his mind then? ^_^
- Another mighty interesting phenomenon regarding Sandbh's approach to thorium is how he somehow still feels the need to apologise for that case pretty often.
| “ | Sticking with blocks alone. That is what most chemistry text-book authors do. They then drill down into the electronic filling sequence, and present the table as Sc-Y-La because it’s not until Ce and Th where f- electrons first make their presence felt. (An objection can be raised to Th d2s2. Still, the presence of ~0.5 of an f-electron is indicated in the solid). And we know the split d-block doesn't become over-visible due to the predominance of the 18-column form. ... Sandbh (talk) 06:12, 8 July 2020 (UTC) | ” |
- So, "f-electrons first make their presence felt" at thorium. In order to justify that statement, he has to "drill down" in his words away from the supposedly highly successful approximation of the ground-state gas-phase electron configurations, because the first 5f electron appears in those configurations only in protactinium. (Dropping those is actually sensible, since you couldn't get further from a chemical environment than looking at one atom all by itself, naturally.) But note how he then doesn't do it for lanthanum and actinium, never mind that the presence of ~0.2 of an f-electron is likewise indicated in solid lanthanum (fcc state, metastable at STP, cite Glötzel). And La metal f band occupancy is between those of Ac and Th (cite Dimitris A. Papaconstantopoulos' Handbook of the Band Structure of Elemental Solids). Any way you look at it, La and Th are still in exactly the same situation. However thorium is allowed into the f block and lanthanum and actinium are adamantly being refused entry.
- Meanwhile, Lu and Lr are given VIP passes to the f block despite having 0 valence f-electrons in their ground-state gas-phase configurations, ~0 of an f-electron in the valence bands and 0 f involvement at all in chemical compounds. Allow me to express puzzlement about why shenanigans at the start of the block are important and those at the end are somehow not raised. Could it be another double standard? Why yes, I think so.
- Either that or he claims that "similarities with Ce" trump the requirement for the right configuration, in which case one wonders what is wrong with La in the f-block considering that the 4f elements are literally called lanthanides:
| “ | From what I've read, the consistency question seems to come down to the treatment of gas phase configurations. So Al stays in the p-block. And Ce starts the f-block. In the case of He its nobility trumps configuration consistency. In the case of Th its similarities with Ce trumps the configuration requirement, with some organisational tidiness thrown in for good measure. ... Sandbh (talk) 04:42, 24 June 2020 (UTC) | ” |
- Perfect, let's sometimes go this way, sometimes go that way. Sometimes A shall trump B, sometimes B shall trump A. All with no justification for why A or B is chosen in any case. To the point that one may start to suspect, like Jensen, that the only real criterion involved in this argumentation is that La under Y is supposedly the standard form and therefore anything else is ganz verboten. Never mind that, as I've demonstrated, La under Y is not even the standard form in any widely accepted sense of the word.
- I think that my decree of Master of Science in computational mathematics allows me to share my own impression of what mathematics is like. Not everything in mathematics is based on logic. Logic itself deals with implications, but to get anywhere, you need some basic presumptions; they are called axioms. You often need to agree on those first, since different people will tell you they want different things. You can make an efficient engine but then it will turn out it needs a lot of fuel, or if that's not a problem, you'll also need it not to break too quickly. Sometimes such choices turn out to be rather voluntary in the end because there's also an assumption a person who creates a model might be missing something and things can go poorly in the ways they're not expected to be, and in estimating what that quantitatively means, you need---you guessed it---your own judgment. There's this pure mathematics free of this voluntarism (and even that is relative: you always need this implicit decision like "what's this idea of 'sequences,' perhaps there is some use in exploring it. What if I invent this idea of 'convergence'"... and so on. By standard, we say that there isn't a finite sum of 1 + 2 + 3 + ..., but there is the idea that it could be -1/12; what's that idea of what is "standard" if not an implicit choice? choices are everywhere, whether they are visible or not) but in the end, when it comes down to decisions, deliberate choices are always made at some point. It may be easier to think that mathematics is our human invention, too; there is no such thing as a linear manifold without a person to conceptualize it first.
- I'm not a huge fan of the criterion Sandbh picks; that being said, it is still a valid criterion even if it's not logically perfect (there's not going to be a logically perfect criterion). You can argue there is a better criterion (in the end, the choice of criterion will be deliberate), just remember that logic alone won't cut it.--R8R (talk) 17:19, 19 July 2020 (UTC)
- +1 Sandbh (talk) 23:10, 19 July 2020 (UTC)
- I decided some clarification might be needed to this; I don't want this idea to be missed even if Sandbh has already said something to this effect.
- By saying that I'm not a fan of the criterion Sandbh has picked I mean that I think he finds most reasonable. I think that after enough drilling down you can say that it is pretty much to his liking. That's fine as far as I am concerned. It's not much to my liking, because I think it inevitably focuses on the beginning of a block whereas I would assume both the beginning and the end are important; that's what it would be like to my liking, I realize that my arguments drills down to that, too. Sandbh's point of view is not of paramount importance and neither is mine; with this in mind, we would still have to coexist somehow in this argument, and I think this realization could be the key for that. It's okay if he doesn't like the things that I like or if I don't like what he does as long as we're both acting reasonably about it. If this really were an argument between the two of us (which I don't consider it to be), I'd be fine with the idea I could try to see where he is coming from, and if it still doesn't help me, I'd say he is very free to do his own thing and not at all be bitter about it. It's up to me, I would say, to formulate my arguments in a manner that would appeal to the other people and not compromise myself (before myself rather than anyone else) while doing that. It's not common that I get into arguments, and when I do it feels like I'm getting flamed on pretty quickly. But even when angry, I still try to think whether I'll regret my words tomorrow or in a year.
- Sandbh has said he tries to argue to the best of his abilities. I believe him, that's as much as you can ask for anyway. And even if it feels like the best of his abilities could be better, saying that out loud would require a lot of confidence on my own part. Could I really claim that I am not blinded by something and that there's nothing to prevent me from saying that the best of my abilities is better than the best of his and that saying that wouldn't expose my flaws and backfire instead? Or given that he tries his best, even if I disagree with him, how should I behave around it?
- I don't quite think that status quo is as much worth preserving as Sandbh hints it is. That being said, the world won't end if it does end up staying what it is. It'll be up for me to accept it gracefully and move on.--R8R (talk) 19:02, 19 July 2020 (UTC)
The status quo
In the ordinary course of life, the "status quo" or existing state of affairs becomes unfashionable or out of date when something better or more attractive comes along and is widely taken up by the masses.
The La form is is popular, and simple to teach, being informed by real electron configurations rather than idealised ones. Thus:
- "The fifth row starts with cesium and is followed by barium and lanthanum. Lanthanum starts another transition series. The succeeding members of this series are hafnium, tantalum, tungsten, etc. Between lanthanum and hafnium, however, an inner transition sequence occurs. The filling of this inner shell (under several outer shells of electrons) constitutes the rare earth elements starting with cerium and concluding with lutetium." (Kleber & Love 1963)
Now, popularity doesn't mean necessarily mean it's right. And some people have argued Lu looks more right in Group 3. Arguments have also been made in favour of La. There are less of these as it has never been considered worth the bother.
As I am accused of sticking my fingers in my ears, so to do the Lu supporters here do, in respect of what Jones (2010), and Schwerdtfeger, Smits & Pyykkö (2020), have to say about the matter. I'm sure by now you're sick of seeing these two:
- "Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics."
- "Although H and He clearly separate from the rest of the PTE, almost every chemist agrees that we can leave these elements in their current place in the PTE, keeping their distinctive quantum nature in mind."
- "Fuzzy concepts like chemical similarity often lead to unnecessary disputes concerning the PTE."
Quite so, say I. The contents of chemistry journals are not replete with a vocal mob of Lu agitators. There is no groundswell of opinion noting the fundamental superiority of Lu. Chemical societies around the world are not being bombarded with a bevy of Lu change proposals.
It's ironic that I say this having been quite active within our own project at attempting to bring clarity to various categorisation schemozzles within the literature. That is not the case here. Sandbh (talk) 00:23, 20 July 2020 (UTC)
Being deeply wrong (or not)
- @Дрейгорич, Droog Andrey, R8R, ComplexRational, Officer781, and DePiep: And let's see who else thinks that something is deeply wrong with the arguments Sandbh is employing here. Double sharp (talk) 03:30, 14 July 2020 (UTC)
@Double sharp:I find your response entertaining in a "stupefied-feeling-of-'really'(?)" kind of way.
You get an impressive 18 out of 20 for managing to present the following five types of argument…
- [1] They must be wrong, of course
- [2] A zombie that will not die
- [3] Drill-down obfuscation
- [4] Conflationary fallacy
- [5] Obfuscation by irrelevant extension (5 marks)…
…and ignoring nine of nine principles governing the arrangement of the periodic table (9 marks)…
…and discrediting the three of three PhD's that reviewed my FoC article (3 marks)…
…and discrediting the IUPAC project team's literature survey (1 mark).
Overall assessment: "Could do better." --- Sandbh (talk) 06:00, 14 July 2020 (UTC)
- I'm not interested what you think. I'm interested what everybody else thinks. I also find it mighty interesting how you're apparently allowed to find my responses "entertaining", whereas Dreigorich is apparently not allowed to feel the same way and LOL at them. Double sharp (talk) 09:09, 14 July 2020 (UTC)
- I was submitting my edit when the internet connection reset and I have to retype it. Anyway, I do find this exchange hilarious, although it has become a bit ad hominem if I dare say. Sandbh appears to be arguing that Ac and Th are somehow so different from each other that they need to be treated separately, despite the same principles involved in both. This breaks Occam's razor - do not make two explanations where one will do. Also, just because something is true "because I said so" doesn't mean it is. Case in point - geocentricism. When it was rejected to make ephemeris generation easier, there still were people who clung to the old ideas "because I said so". Is Sandbh one of them? ― Дрейгорич / Dreigorich Talk 10:05, 14 July 2020 (UTC)
- @Дрейгорич: My apologies if anything I wrote could be considered ad hominem attacks, BTW. Please do tell me if you think I have gone overboard somewhere and I'll strike the remarks out.
- (BTW, regarding Lr in a Lu table: that's not a problem for me because it is not a 7p element but a 6d one. The ground-state electron configuration anomaly means nothing for real chemistry, in which lawrencium is very happy to use 6d orbitals. That's how I resolve the problems of delayed starts in La-Ce, Ac-Th-Pa, Lr-Rf, and elements 121-126 all in the same way.) Double sharp (talk) 14:56, 14 July 2020 (UTC)
- I think it's just frustration. No need to strike anything out. ― Дрейгорич / Dreigorich Talk 15:04, 14 July 2020 (UTC)
- I was submitting my edit when the internet connection reset and I have to retype it. Anyway, I do find this exchange hilarious, although it has become a bit ad hominem if I dare say. Sandbh appears to be arguing that Ac and Th are somehow so different from each other that they need to be treated separately, despite the same principles involved in both. This breaks Occam's razor - do not make two explanations where one will do. Also, just because something is true "because I said so" doesn't mean it is. Case in point - geocentricism. When it was rejected to make ephemeris generation easier, there still were people who clung to the old ideas "because I said so". Is Sandbh one of them? ― Дрейгорич / Dreigorich Talk 10:05, 14 July 2020 (UTC)
@Double sharp: I know you're interested in what I say, since you respond to my contributions. By "entertaining" I mean in an intellectually engaging way. In "marking" your response, I forgot about you ignoring nine of nine of the environmental factors mentioned earlier. So a more accurate grade is 27 out of 29.
Ac and Th
@Дрейгорич: I've never said Ac and Th are somehow so different from one another that they need to be treated separately. Rather, at perhaps the simplest level, I've observed that a block starts upon the first appearance of the applicable valence electron: s at H; p at B; d at Sc; f at Ce. These four are the survey markers for the structure of the rest of the PT, consistent with the "no fuss" example I cited of Kleber and Love (1963). Some related observations are:
- In electronic structure terms, lanthanum appears to have the advantage of incumbency, since the 5d1 electron appears for the first time in its structure whereas it appears for the third time in lutetium, having already made a brief appearance in gadolinium (Trifonov 1970, p. 201–202);
- Lavelle's original objection (1983) still holds: La and Ac in the f block would represent the only case of a vertical pair of elements with incongruous electron configurations;
- "For the purpose of selecting an optimal periodic table we prefer to consider block membership as a global property in which we focus on the predominant differentiating electron." (Scerri & Parsons 2018). Here, an La table has fewer differentiating electron discrepancies.
Double sharp has argued that:
- Condensed phase electron configurations are more relevant. In fact, they make the sitation worse. In an La table, the f-block has 11 discrepancies; in an Lu table the f-block gas 28 discrepancies.
- Differentiating electrons cannot always be discerned. For example Z = 40 Zr is 4d2s2 and Z = 41 Nb is 4d45s1. Here the d/e seems to be d2s1. In fact, in such cases the d/e is taken to be the newly added d-electron, rather than the s-electron that was already there (so to speak).
- Differentiating electrons make no difference to the chemistry of the elements and are therefore irrelevant. In fact, a counterexample is silver, the d/e of which is expected to be a d-type, but in fact turns out to be an s-type. Silver then acts predominately as a main-group metal rather than a transition metal.
To discredit my d/e argument, Double sharp engages in drill-down obfuscation, as follows:
- "The differing behaviours of group 11 metals are simply explained from the relative energy differences between (n-1)d and ns (the chemically active valence sub-shells) at the end of the d-block. In Cu the gap is not so big, we see +1 and +2 states frequently. In Ag it is bigger, in Au it is smaller again because of relativistic destabilisation of 5d. That's what controls the ionisation energies and hence common oxidation states, not the differentiating electrons."
Whereas I say:
- "The level of detail you enter into is not needed. I agree with Scerri who believes there is great merit in taking as philosophical, and as abstract as possible, an approach to the periodic table. It's not necessary to drill down.
Friedrich, a physicist, has written on this:
- "Exact sciences cherish approximations. [italics added] More often than not, resorting to approximations is a matter of necessity…that is the case when a problem cannot in principle be solved exactly. For instance, many-body problems fall all in this category, whether they are classical or quantum…We note that here many means more than two; hence there are very many many-body problems. Approximations are also introduced when seeking a qualitative understanding of a problem: approximations (called in this context models or treatments) reveal the structure of problems and aid in identifying analogies with other problems, thus adding to the sense that we can make of them…
- "Approximations to the solution of a certain problem form a hierarchy, according to the degree of their accuracy; for many problems, an arbitrary accuracy can be achieved in principle and often in practice. A good example is the N-electron atom problem."
On Occam's razor, and as I said, "Some care is required wrt Occam's Razor. It's not universal. It relies on classical logic which does not necessarily hold when comes to arrangement of the periodic table. If it did then there would be some kind of proof for resolving the Group 3 question when there isn't. Simplest sufficient complexity is more in keeping with the philosophy of the PT."
Finally, I have never asserted something is true "because I said so". Rather I have noted the popularity of the La form, as mentioned by Mathias (1969); Myers, Oldham and Tocci (2004, p. 130); Clarke and White (2008); Lavelle (2008; 2009); and the IUPAC Group 3 project (2019).
Double sharp instead points out that quite a few people who look at the question conclude in favour of Lu in group 3. His own arguments are based on classical logic.
In fact the predominance of these people did so on the basis of looking at one property. The few exceptions are Jensen; Holden; and Horovitz and Sârbu; and Alvarez. Jensen took into account several properties. Unfortunately he was selective in his choice of arguments. Holden looked at seven properties/arguments the strongest of which was that of Landau and Liftshitz. Unfortunately L&L's argument has been misinterpreted and doesn't stand up under close scrutiny. Horovitz and Sârbu supported Lu but this was on the basis of 18 mainly physical rather then chemical properties. Alvarez (2020) supports lutetium on the basis of trends in atomic size, coordination number, and relative abundance of metal–oxygen bonds. However, the trends involved apply regardless of whether lutetium is under Y or at the end of the f-block, after Yb.
Scerri (2020, p. 381) opined, and I agree, that the matter cannot be resolved using on the basis of comparing physical (including spectroscopic), chemical, and electronic properties and trends, since the two options are effectively indistinguishable.
I advocate taking more of a helicopter view. This means examining Group 3 in the context of its surrounds; the congruity of the f-block; patterns seen elsewhere in the periodic table; the periodic law; and global considerations. I aim to be systematic in that my arguments are interlocking, consistent with the facts and parts of the periodic table as an integrated, complex structure (Scerri 2012a, pp. 282–283). I argue that fuzzy logic plays a part here, too. That is consistent with what Scerri recently said:
- "We should accept that a degree of convention must be used [Sandbh italics] in selecting a periodic table that can be presented as perhaps the best possible table that combines objective factors as well as interest dependence."
If I've misrepresented Double sharp I'm sure he'll let us know. --- Sandbh (talk) 03:01, 15 July 2020 (UTC)
- The start of the block is a bit annoying and is largely the source of the controversy I'd say. La and Ce are both exceptions to Aufbau - La should have one f electron, while Ce should have two. The rest of the f block (save Gd) is regular. Pr has three (4d3), Nd has four (4f4), Pm has five (4f5), and so on until Yb at fourteen. The early actinides follow the same principle. Ac should have one, Th should have two, Pa should have three, and so on. It's the later actinides that confirm the pattern, and Lr. Lr is so different from Es-No that it doesn't make sense to put them together. Lr is more like Rf-Hs than it is to Es-No. From here, we observe the table has two splits - one between Ba/Ra and La/Ac, starting the f block, and one between Yb/No and Lu/Lr, ending the f block. ― Дрейгорич / Dreigorich Talk 03:09, 15 July 2020 (UTC)
- @Дрейгорич: Yes, agree about the start of the block. La and Ac are not exceptions; there is no fundamental reason why they should follow Aufbau, since Aufbau has no first principles derivation.
- In their trivalent ionic forms, Ce to Lu show perfect regularity in their f-electron numbers. So, Ce3+ is f1, Pr is f2, all the way up to Yb f13 and Lu f14. This sequence also corresponds to the f-electron caused Ln contraction, first seen in Ce, last seen in Lu.
- Metallurgically speaking, Lu closely resembles Er and Ho.
- The break is between La (no f electron) and Ce (first appearance of an f electron). At the other end the break is between Lu (culmination of the f-electron induced contraction) and Hf with the resumption of filling the 5d sub-shell (La is 5d1 as are Gd and Lu).
- In their trivalent ionic forms, Pa to Lr show perfect regularity in their f-electron numbers. The one exception is Th which is d1 rather than the expected f1. As per your Gd note, this is anomaly not worth losing any sleep over.
- Lr is not so different from Es-No. The most stable oxidation state for Es, Fm, Md and Lr is +3; No is the exception, being +2 (it still shows +3 too, however). The volatility of LrCl3 is similar to that of the chlorides of Cm, Fm and No, and much less than that of Rf.
- For the An the break is between Ac and Th since Th is the congener of Ce. This results in anomaly since Th has no f electron. While this is an anomaly not worth losing sleep over, the consolation prize is that Th metal features the first appearance of a 5f electron (~ 0.5). This f-presence is sufficient to cause Th to adopt an fcc structure rather than the hcp structure of the group 4 metals. At the other end, the break is between Lr and its similarity to the late heavy actinides; and Rf which flags the resumption of filling of the 6d sub-shell (Ac is d1; Th is d2; :Pr-Np and Cm are d1).
- In this light, could you please reconsider your position. --- Sandbh (talk) 13:26, 15 July 2020 (UTC)
- I'm doing a Double sharp and giving up. I'm not engaging with you anymore after several months of this. ― Дрейгорич / Dreigorich Talk 19:04, 15 July 2020 (UTC)
@Дрейгорич: I see you gave some reasons for Lu in group 3. When I explained your reasons are not supported by the properties involved, you give up. And that's fine by me. Looking back at your contributions, I saw you started from pure Aufbau. From there you focused on anything consistent with pure Aufbau. The thing about pure Aufbau is that there's no such thing. The second is that it has no first principles derivation. In a universe with different fundamental parameters it's conceivable Aufbau would work perfectly. In our universe it doesn't. So the question is do we rely on something that doesn't exist (pure Aufbau) or do we start with how things really work in our universe?
If your preference for Aubau and Lu is based on a personal liking of regularity or symmetry that's fine. Nothing I say is likely to change that.
Just bear in mind what Imyanitov (2019) said:
- "If one seeks for the maximum chemical utility…[one] should opt for the more 'unruly' tables. If one seeks maximum elegance and orderliness above all…[one] should favor the more regular representations."
- Imyanitov N.S.: Spiral as the fundamental graphic representation of the Periodic Law. Blocks of elements as the autonomic parts of the Periodic System. Found. Chem. 18, 153–173 (2016)
--- Sandbh (talk) 04:58, 17 July 2020 (UTC)
Surprisingly enough, the reason I actually respond to Sandbh's contributions is not because I actually consider them a serious threat to my arguments at this point. They are just obviously bad by the classical logical paradigm that everybody in science follows, never mind Sandbh sticking his fingers in his ears. I am also not responding to them because I think they will convince him. In refusing to accept classical logic he has blasted himself off onto another planet already. I simply respond to them to convince others reading this about that, because they have not yet had the dubious pleasure of arguing with someone who clearly never used logic to get to his stand in the first place, and may be a bit more inclined to assume good faith that there is some logic being involved here.
But indeed, Sandbh misrepresents me. That's another reason why I still respond. Perhaps I should stop, because arguing with someone who doesn't use logic merely brings you down to that level. But, against my better judgement, here is one last try. I do not advocate condensed phase electron configurations alone. In the solid state, there are no pure atoms, there are no pure configurations, everything would be an anomaly with fractions everywhere.
- Sandbh: I object. At no point did I say you advocate condensed phase electron configurations alone. I said, "Double sharp has argued that condensed phase electron configurations are more relevant."
What I advocate is simply considering configurations across all chemical environments. Just see how many valence electrons there are, and which orbitals they go into, and which orbitals participate in the bonding. And just ignore the chemically totally irrelevant anomalies.
- Sandbh: This is a level of detail that isn't required. One only needs to consider the most important orbitals.
It is lamentably expected at this point to see Sandbh referring to silver in a bid to prove those irregularities relevant. Palladium in the gas phase also has that prematurely closed 4d shell. You don't see any main group character from it. Gold also has a prematurely closed 5d shell. Unlike silver which prefers a main-group-like +1 state, gold prefers a firmly transition-like +3 state. Roentgenium has a different configuration to gold in all likelihood, yet it should behave very similarly to gold. Chromium and molybdenum have the same configuration, different to tungsten, yet actually molybdenum and tungsten act very similarly and it's chromium that's quite different. Maybe in Sandbh world it's permissible to trumpet the configuration only in that one measly case of silver where it gives you the answer you want, but everywhere else this is called a double standard.
- Sandbh: A nice paper tiger argument. And just why would we expect Pd to have main character in the first place, given its d10 configuration?
The same thing happens if we look at differentiating electrons. So, Sandbh notes that Ag has a 5s differentiating electron, Au has a 5d one. This supposedly explains the predominantly "main-group" character of Ag as opposed to that of Au. Well: Tc has a 5s differentiating electron, Re has a 5d one. I am not sure in what sense of the word technetium with its extremely easy interconversion between lots of oxidation states may be described as more "main-group" than rhenium, but I am confident that Sandbh can invent a way. That is, if he doesn't then proceed to call this a "conflationary fallacy", which is the name in Sandbh world for what science generally uses classical logic to call a falsifying counterexample.
- Sandbh: Obfuscation by irrelevant extension.
That is exactly why, in order to rationalise the differing behaviour of Cu, Ag, and Au, I have done what Sandbh calls "drilling down". They behave very differently, but Sandbh cannot tell why in the case of Cu vs Au, because according to him they both have a d differentiating electron. Only with looking at energy levels you can see why. Actually all of Cu, Ag, and Au can show various oxidation states, but the energy gaps explain which ones are likely to be more stable. Of course that's not complete in itself, you must also look at lattice energies etc. But that's the only way that won't get torpedoed by lots and lots of counterexamples. And of course you get massive bang for your buck looking at energy levels, because that's the entire reason why the periodic table fills up in the order we all know.
- Sandbh: Drill down obfuscation.
- On the basis of spectroscopy, we observe, collect and exploit empirically derived patterns in the arrangement of electron configuration—within the scope of the the independent-electron approximation, one of the central paradigms in modern chemistry and physics.
- Chemists use these patterns to generate simple rules associated with stable (and by inference, unstable) atomic, molecular and ionic electronic configurations: full shells are associated with stability, whereas partially filled shells unstable and therefore reactive.
- Electron configuration theory promotes the rules and patterns, but makes no attempt to explain how or why the magic numbers of electrons associated with the patterns arise [it cannot]. Nor can electron configuration theory explain anomalous behaviour…except by invoking a new pattern, which it readily does.
- With electron configuration theory, and its collection of ideas, the pattern is the science. Electron configuration theory has been taking this highly successful approach, despite its characterisation as a first order approximation, for more than 100 years.
- NOTE: My response here is adapted from a discussion Mark Leach gives about modern Lewis theory.
Sandbh's theory is not good enough to explain anything outside the examples he likes trumpeting. To rescue it he resorts to calling out supposed "conflationary fallacies" that result when you extend his arguments. He forgets that the periodic law is a statement of recurrence of chemical and physical properties as a periodic function of atomic number. It does not have any footnotes attached to it restricting it only to some parts of the table. If a supposed principle of building up the periodic table cannot meet this standard, as his block start rule doesn't because of thorium, it must be thrown out. Not for him though.
- Sandbh: While the PT may look like a model of organisation, harmony and efficiency, consider how many of its inconsistencies nearly all chemists don’t lose any sleep over.
The funny thing is that although he doesn't hold himself to this standard, he tried to hold me to it. Just look at him asking me below if Ca, Sr, Ba, and Ra would have to be called transition metals because of their d involvement. Never mind me having explained from first principles why the s block is different, which is a precondition of legitimacy of my approach by classical logic. Of course, that's classical logic and is lost on him. Below I have tried using his approach: "you can't extend my argument to the d block, it's a conflationary fallacy". I was hoping that once he banged his head against the wall fruitlessly trying to refute that he just might start to understand the need for classical logic. OTOH he simply said "well done", thinking that I'd been converted to his weird ideas. As maybe I should have expected, as this sort of demonstration is far too close to reductio ad absurdum for it to possibly work.
Sandbh also is strangely referring to the congruity of the f block. Yet he fails to see the need for the congruity of the d block. That's why he fails to understand the trends Alvarez pointed out. He only focuses on Lu in the f block, and he doesn't bother to see how horrid La looks in the d block. Sandbh fails to see that in every property La and Ac are massive outliers in the d block but are perfectly normal for the early f block. He also fails to see that Lu is perfectly normal for the early d block and that, as Dreigorich has so kindly relieved me of the need to say, Lr is a massive outlier in the late f block but perfectly normal for the late d block. The data has all been presented to him, but he doesn't see it. To him the gospel truth seems to be Restrepo, who amusingly enough considers stoichiometry, ergo will never find any such thing as similarities between the d elements. Only the 4f elements. And he can't even find the usual wrong-valence lanthanides Ce, Sm, Eu, Yb all as exceptions. So much for Restrepo.
- Sandbh: Alvarez (2020) supports lutetium on the basis of trends in atomic size, coordination number, and relative abundance of metal–oxygen bonds. However, the trends involved apply regardless of whether lutetium is under Y or at the end of the f-block, after Yb. User:Dreigorich has demonstrated no such thing, aside from referring to non-existent breaks between Ba-Ra and La-Ac, and between Yb-No, and Lu-Lr. Yes, I do take note of Restrepo, given his work with Scerri, his many peer-reviewed publications, and that fact that he is a member of the IUPAC group 3 project team.
This is where Sandbh will come running saying I'm engaging in a "they must be wrong" argument, never mind that that is not a fallacy if you have an actual argument why they are wrong.
Anyway, we have a consensus for Lu, classical logic is as Sandbh remarks behind Lu. If I went out and changed it right now with this 7-1 consensus, WP policy would be behind me. I only go for an RFC because that's how it was changed in the first place and so that those who understand WP policy have nothing to say.
- Sandbh: WP policy would not be behind you. I need only point to the 4:1:1 predominance of the La form, as reported by IUPAC. I can add the fact that, as noted, several authors have commented to the effect that La-Ac remains the most popular form. Mathias (1969) grumbled about it. Myers, Oldham and Tocci (2004, p. 130) found La-Ac was the most popular form of periodic table, a sentiment echoed by Clarke and White (2008); and Lavelle (2008; 2009). IUPAC approved a Group 3 project to address the question and you propose to gazump their findings. Seemingly you have some special insight into their workings.
- Our personal opinions carry no weight in this context. We are an encyclopaedia that reflects the literature, not an independent research forum.
For those who don't understand WP policy, as Sandbh weirdly demonstrates by referring to three cases where counting the literature alone without looking at arguments shows it's not for a change we made (group 12 exclusion from transition metals is 50-50, metalloids in literature are more likely to include Po but not At, nonmetals indeed needed a discussion) – nothing will help them.
- Sandbh: I understand the WP policy fine, having run up against it several times. Group 12 exclusion from TM was based on a consideration of the literature, given the literature was 50/50, as discussed within our project. Metalloids in the literature are likely to include B, Si, Ge, As, Sb, Te on an average of 93% of the time. Po is 48%; At is 40%, average 44%. We discussed the question of Po and At in this project, in this light, and again on the basis of what the literature had to say about Po and At. We discussed nonmetals in this project on several occasions, and concluded the literature is a shambles, so decided on just reactive nonmetals and noble gases. There is nothing weird about these three outcomes in the context of WP policy.
Lu has too much science and too much of a consensus and too much of the agreement of the relevant sources behind it to lose.
- Sandbh: No, there is no such (good) science, consensus, and agreement.
- The majority who examine the issue don't examine the issue. Rather they usually examine one property. They only person who attempted a comprehensive examination of the issue was Jensen, and he was criticized by Scerri (chair of the IUPAC project group), for being too selective in his arguments. There was Holden (1985) who looked at seven properties but estimated the argument of Landau & Ligshitz (1958) to be the strongest. I've pointed out the shortcomings of L&L earlier.
- There are still plenty of arguments supporting La, as set out in our IUPAC submission (which you disavowed himself of). There will be plenty more in my article, when my article is published on line.
This is going to be my last contribution here. I am drafting the RFC. Unfortunately I will have to waste more time arguing with Sandbh at that RFC, but the possibility to finally get this mistake corrected is worth far too much to give the idea up. And, since it will show exactly what I have to deal with to a much wider range of people, I am confident that logic will win the day. Double sharp (talk) 03:51, 15 July 2020 (UTC)
- Sandbh: There is so much more to the question than classical logic, including: the conservative nature of the chemical establishment; history; reputation; the philosophy of chemistry; organisational politics; popular opinion or the silent majority; the nine principles governing the organisation of the PT; and the 28 things nearly all chemists don't lose any sleep over. Sandbh (talk) 02:32, 16 July 2020 (UTC)
Lanthanum at high pressure
@Double sharp: In this article the authors note that La does not have any electrons in the 4f shell. They go on to say that, “Theoretical studies by Herbst (1992) suggested that, around 1100 GPa, some electron occupancy in the 4f band of La, can be expected.”
Now, if La metal is supposed to have some 4f hybridisation at SATP why would it take a whopping 1100 GPa before some 4f occupancy could be expected? Sandbh (talk) 11:53, 13 July 2020 (UTC)
- Because, surprisingly enough, that's not what the study by Herbst says (10.1103/PhysRevB.46.6665). That's the whopping great pressure needed to make La become totally 4f1:
| “ | ...a large pressure, ~11 Mbar, is required for a 4f0–4f1 transition. Furthermore, the 4f level width at that pressure has increased by an order of magnitude over its normal-pressure value, which indicates that prior to such a 4f configuration change a transition from localized to itinerant 4f magnetic moments is to be expected. | ” |
| — Herbst, J. F. (1992). Lattice-constant dependence of the 4f excitation energy in lanthanum metal. Physical Review B, 46(11), 6665–6670; italics mine | ||
- We may easily get some hybridisation into 4f beforehand that explains the melting point even at room temperature. Double sharp (talk) 12:00, 13 July 2020 (UTC)
@Double sharp: In this context, I think the difficulty is what Mathias said, here doi:10.1007/978-1-4684-3351-7_31 (p. 481):
- "One more comment, Zachariasen measured the number of f-electrons in lanthanum. That can be done without my great problem, as far as he's concerned, by determining the size of the radius, and he came to the conclusion that the concentration of 4f electrons in lanthanum can under no circumstances exceed 0.05 of the net f-electron per atom."
Now, I have no issue with recognising Th metal as having ~½ an f-electron. But La metal with 19/20ths of a d-elecron, being counted as an f-block metal with ≤ 1/20th of an f-electron strikes me as a block too far.
In another article by your three-cheers fave, Mathias, 1970, "Superconductivity and the Periodic System: The breakthrough leading to superconductivity at 21°K is shown to follow simple rules to be found in the periodic system", American Scientist, vol. 58, no. 1, pp. 80-83, here, he writes:
- "Looking at the periodic system gives a picture, if only a qualitative one…The…f elements [Ce-Yb] are those where the f electrons are well localized, though more for the 4f series than for the 5f series [U-Lw]. However, the elements preceding these series, such as barium, lanthanum, [italics added by Sandbh]…while not yet having a definite f occupation, seem to anticipate it, judging from their metallurgical behaviour. Probably this anticipation takes the form of hybridization in their electron configurations, as it has long been known that at the beginning of the f series the electron configurations are extremely pressure-sensitive."
Here, Mathias refers to Ba and La as preceding the 4f series, and at the same time suggests they seem to anticipate f occupation. Once again, this supports treating La as incipient f-block metal, rather than a genuine f-block metal. Placing La in group 3, with La having ≤ 19/20ths of a d electron and ≤ 1/20th of an f-electron, and counting it as an incipient f-block metal, seems entirely apt. Sandbh (talk) 04:08, 14 July 2020 (UTC)
- When it comes to lanthanum, with some definite involvement of 4f in the metal (I remind Glötzel's later 1978 figure of ~0.17 electrons in the 4f band), and with significant 4f-5d hybridisation in its compounds that is exactly the same as for cerium(IV), Sandbh wants to exclude it from the f block. When it comes to lutetium, with 0 valence electrons in the 4f band, and completely localised 4f orbitals in all compounds, Sandbh wants to include it. Double sharp (talk) 04:38, 14 July 2020 (UTC)
@Double sharp: I think it's telling that you have no comeback on Mathias, and can only hope to distract the audience by drawing away their attention to Glötzel. In do doing you engage in a type [3] drill-down obfuscation argument. Here's something else Mathias said:
- "At this point let me remind you that when looking for a superconductor we may look at the electron configuration of the isolated atom[!] [my exclamation]. But configuration is certainly different in the actual crystal typical for the element or its compound. Clearly it is a rather crude approximation, but since it works, [my underline] it may not be too bad an approximation."
Once again we see that free atom configurations are the consideration. Drill-down obfuscation into solid state physics to resolve the group 3 question is not required, as per the nine principles, which you conveniently choose to ignore. All the authors involved are wrong of course, per a type [1] argument. Sandbh (talk) 07:11, 14 July 2020 (UTC)
- Never mind that Sandbh himself admits that it doesn't work every time he justifies thorium in the f block by its solid-state configuration. And also that strangely enough, Sandbh thinks "it's telling that you have no comeback on Mathias, and can only hope to distract the audience by drawing away their attention to Glötzel", when Glötzel refutes what Mathias said, and Sandbh literally last post had no comeback to Herbst, and instead drew our attention away to Mathias. Double sharp (talk) 08:58, 14 July 2020 (UTC)
- For my "comeback" on Herbst, see here. Sandbh (talk) 06:41, 18 July 2020 (UTC)
The Journal of Rare Earths: music to my ears
From 10.1016/S1002-0721(17)60984-0, a 2017 paper. My bolding throughout.
| “ | From a traditional synthetic viewpoint, lanthanides are considered as hard Lewis acid with 4f electrons shielded from the external perturbations by the 5d and 6s shells, therefore, 4f electrons are considered not involved in the chemical bonding. However, some systems (e.g., lanthanide trihalides) have shown pronounced 4f hybridization indicative of covalent bonding interactions[28,40,41]. Orbital hybridization can determine how many bonds an atom can form and the geometry of molecules. The number of hybrid orbitals formed depends on the number of electrons occupying the outermost orbitals, or the so-called valence shell. Using first principles theory, Strange et al. found that there are two types of f electrons: localized core-like f electrons that determine the valency, and delocalized band-like f electrons that are formed through hybridization with the s−d bands and which participate in bonding[28]. Later, a pronounced 4f hybridization was found for LuF3 using three different relativistic methods of calculation, demonstrating the participation of 4f electrons in chemical bonding[40]. Despite the observation of orbital mixings of Ln(4f) in LuF3, Wang et al. pointed out that they are not evidences of bonding[42,43]. In this work, we start from the orbital hybridization to study the chemical bonding characteristics of 4f electrons in RE compounds. | ” |
So, Lu isn't using its 4f electrons for bonding. You have to look at the hybridisation instead.
| “ | In previous study of the chemical bonding nature of RE compounds, the bonding in f-element compounds has been described as d sub-shell participation. According to the Pauling approximate energy level diagram and Cotton atomic orbital energy level diagram, it can be found that the 5d and 4f sub-shells have very similar energies. Therefore, those 4f electrons can also go to 5d orbitals like Ce, Gd and Lu, as shown in Table 2. Owing to the similar energies between 5d and 4f sub-shells in RE atoms, the hybridization probability between two types of o[r]bitals is larger. Compared with 4f energy level, the position of 5d energy level is more sensitive to the surrounding crystal field. That is, the position of 5d energy level of RE cations will be modified by selecting different ligands. This will create conditions for orbital overlap between 4f and 5d orbitals, and the f orbitals also have the probability to participate in chemical bonding via hybridization with the other sub-shells in RE atoms, such as 4f, 5d, 6s and 6p. | ” |
Could it be? Exactly the view of lanthanum through ytterbium as [Xe] (4f 5d 6s 6p)3–16 I fervently advocate? And what I've been saying about 4f participation?
| CN | Hybridisation |
|---|---|
| 2 | sp |
| 3 | sp2 |
| 4 | sp3 |
| 5 | sp3d |
| 6 | sp3d2 |
| 7 | sp3d3 |
| 8 | sp3d4 |
| 9 | sp3d5 |
| 10 | sp3d5f |
| 11 | sp3d5f2 |
| 12 | sp3d5f3 |
| 13 | sp3d5f4 |
| 14 | sp3d5f5 |
| 15 | sp3d5f6 |
| 16 | sp3d5f7 |
| “ | Orbital hybridization can determine how many bonds an atom can form and the shape of molecules. That is, the coordination number of the central atom in molecule depends on the atomic orbital hybridization. As shown in Table 3, the atom orbital set determines the hybrid orbital set and consequently the chemical bonding type. The chemical bonding types of RE elements with the valence shell 4f0−14 5d0−1 6s2 6p0 are divided into three types on the basis of various hybrid orbital sets, respectively involving sp, spd, and spdf hybridizations. For RE atoms, it is not necessary for all the valence electron orbitals to hybridize. Therefore, the coordination numbers of RE atoms can have a wide range of 2−16. Moreover, with decreasing the 4f electrons in RE elements, the probability of hybridization between 4f, 5d, 6s and 6p orbitals increases due to the similar energies, and the f-block elements with smaller number of f electrons will possess larger coordination number. Which orbitals participate in chemical bonding and how they are distributing among different orbitals can be clarified on the basis of these hybrid orbital sets. Specially, it can be found that the coordination number of RE atoms should be larger than 9 in the case of 4f orbitals that participate in chemical bonding. Consequently, the chemical bonding characteristics of 4f orbitals in RE atoms can be quantitatively related to the coordination number. In the study of chemical bonding of 4f orbitals in RE atoms, the compounds in which the central RE atom with the coordination number is larger than 9 may be selected. | ” |
Oh my. A statement of exactly what I've been saying, following MacKay et al. I do wonder if Sandbh is going to find some reason to claim that these authors writing in 2017 are wrong in order to protect Parish from 1977.
Also note my bolding above. Could it be exactly what I've said about the first few elements preceding the delayed collapse, viz. La and Ce, having the largest f involvements of all the lanthanides?
| “ | In order to identify the chemical bonding characteristics of f orbitals in RE compounds, we will select typical RE compounds in which the central RE cations with nearly empty 4f orbitals, such as La3+ and Ce3+. According to the valence electron structure, we can deduce that 4f, 5d, 6s, and 6p orbitals will participate in the hybridization for La and Ce, while 4d, 5s, and 5p orbitals will participate in the hybridization for Y. We therefore selected La(NO3)3⋅6H2O, Ce(NO3)3⋅6H2O, and Y(NO3)3⋅3H2O as examples. For crystalline La(NO3)3⋅6H2O and Ce(NO3 3⋅6H2O, the coordination number of central RE cations are 11[46]. From Table 3, it can be deduced that two f orbitals participate in RE−O chemical bonding in these two compounds. In crystalline Y(NO3)3⋅3H2O, the
coordination number of central Y3+ is 9.[47] IR spectroscopy serves as a useful tool to provide the chemical bonding vibration frequency of typical groups. For RE(NO3)3⋅nH2O (RE=La, Ce, Y; n=3, 6), the RE−O chemical bonding will influence both NO3− and H2O ligands. We tracked the crystallization process of La(NO3)3⋅6H2O, Ce(NO3)3⋅6H2O, and Y(NO3)3⋅3H2O in aqueous solution by in situ ATR-IR spectroscopy. During this structural transformation process, RE3+, NO3− and H2O ligands undergo phase transition from liquid to crystalline state, and crystalline RE−O bonding was thus formed. By capturing the intermediate structural characteristics of NO3− and H2O ligands, we can obtain some information concerning RE−O bonding in crystalline hydrated nitrates. |
” |
Yes! Yes! Yes!!!!
| “ | By comparing the time-dependent ATR-IR spectra of La(NO3)3⋅6H2O and Y(NO3)3⋅3H2O, the splitting degree of ν3(NO3−) mode in La(NO3)3⋅6H2O is smaller than that in Y(NO3)3⋅3H2O. Therefore, it can be deduced that the participation of f orbital in chemical bonding can produce a more isotropic chemical bonding environment surrounding NO3− group. ... Comparing La(NO3)3⋅6H2O and Y(NO3)3⋅3H2O (Fig. 1), the wavenumber of crystalline H2O with stretch vibration band at ~3400 cm–1 in Y(NO3)3⋅3H2O is smaller than that in La(NO3)3⋅6H2O, indicating the decreased interaction between RE3+ and H2O when f orbitals participate in the RE−O chemical bonding. | ” |
Behold, the actual consequences of f orbital involvement! We can now add that as one of the other things positing f orbital involvement in La can easily explain:
- melting points;
- high coordination numbers;
- heats of sublimation;
- superconductive properties;
- crystal structures;
- molecular geometries of complexes;
- interaction between Ln3+ and ligands;
- isotropy of chemical environment ligands find themselves in.
Just try explaining all of that without f orbitals. It'll all be one argument for this, one for that, and all violating Occam's razor handily.
To be fair, I would want some follow-up information. The other lanthanides ought to be investigated, to observe what happens as the series goes from La to Lu and f involvement dwindles to nothing. But this is already most encouraging.
Now waiting for some creative means from Sandbh to write this off. Double sharp (talk) 05:11, 14 July 2020 (UTC)
- @Double sharp: There are a several things here.
- Did they really mean to say, "Therefore, those 4f electrons can also go to 5d orbitals like Ce, Gd and Lu, as shown in Table 2"? Glasses down! Game over!
- Pekka Pyykko wrote: "The lanthanide contraction of the Ln-X bond lengths in LnX3 molecules from Ln = La to Ln = Lu is partially a relativistic effect. The latest estimate gives 9%–23% relativity, depending on the system. The main valence orbitals of the Ln, forming their covalent bonds, are the 6s and the 5d." The last bit is important. It isn't necessary to drill-down to hybridisation phenomena among the Ln to resolve the group 3 question. On this basis, shall we count the heavy alkaline earths as transition metals?
- I'll ask Pyykkö about that article in which he and his colleagues found a pronounced 4f hybridization for LuF3 using three different relativistic methods of calculation.
- It isn't necessary to drill down to f-electron involvement to address melting points, high CN, heats of sublimation, superconductive properties, and crystal structures. It may be for molecular geometries of complexes; interaction between Ln3+ and ligands; and isotropy of chemical environment ligands find themselves in.
- f electrons tend to be invoked for far more properties than they are actually responsible for, because that's the easy way and doesn't require much deep thinking.
- Some care is required wrt Occam's Razor. It's not universal. It relies on classical logic which does not necessarily hold when comes to arrangement of the periodic table. If it did then there would be some kind of proof for resolving the Group 3 question when there isn't. Simplest sufficient complexity is more in keeping with the philosophy of the PT.
- --- Sandbh (talk) 07:55, 14 July 2020 (UTC)
One really wonders what the difference between "Occam's razor" and "simplest sufficient complexity" is. Occam's razor says "entities should not be multiplied without necessity". In other words, if both can explain the data, a simpler theory is preferred to a complex one. But a theory which is so simple that it cannot explain the data is not acceptable. Ergo, Occam's razor is identical to simplest sufficient complexity.
Faced with an explanation that says "f orbitals contribute for all of this" versus one that tries to explain each thing singly – and failing anyway at the very end for those cubic molecular geometries and needing f orbitals – simplest sufficient complexity (= Occam's razor) gives an airtight case for the first one.
Faced with an explanation that says "let's explain electronic structure by configurations in chemical compounds", versus one that says "let's try to explain electronic structure for chemistry by looking at the configurations of the gaseous atoms in a far from chemical environment, and then in order to save thorium declare that only the first row of the block matters, the rest are somehow totally different" – simplest sufficient complexity (= Occam's razor) gives an airtight case for the first one.
Faced with an explanation that generalises happily everywhere on the periodic table without any need for limits, versus one where all such attempts to generalise are met with claims that it's fallacious to extend the arguments outside their scopes (because once you do that they ask for something chemically silly) – simplest sufficient complexity (= Occam's razor) gives an airtight case for the first one. Don't multiply arguments for various cases when one can unite them all, that's what simplest sufficient complexity is telling you.
Simplest sufficient complexity gives an airtight case for Lu in group 3 and my arguments. Double sharp (talk) 15:08, 14 July 2020 (UTC)
| “ | On this basis, shall we count the heavy alkaline earths as transition metals? ... Sandbh (talk) 07:55, 14 July 2020 (UTC) | ” |
You know, I've explained the s block situation many times here, there's no need for me to explain it again. However, that approach requires classical logic and therefore is not going to be effective on you. So let me try your approach.
"That is not within the scope of my argument. My argument about f involvement works for the f block only. Applying it to the d block is a conflationary fallacy."
Refute that! /s Double sharp (talk) 15:42, 14 July 2020 (UTC)
- @Double sharp: ^_^
- "Simplest sufficient complexity" is a top down approach. It means we look at the broad contours of a sitation and when we have sufficient observations at, say, the 80/20 level, we stop. We acknowledge the 20%, and bear them in mind, but we proceed to make our generalisations at the 80% level.
- Occam's razor requires a (classical logic) drill-down. It looks for the minimum number of assumptions to arrive at complete consistency. An 80/20 approach will not do. It's 100/0, or bust.
- Cubic molecular geometries do not require f orbitals, per Parish.
- The old horse of gas phase v solid state electron configurations, and "Saving Private Thorium" is type 3 drill-down obfuscation, and a type 2 zombie that will not die.
- I see you cannot address the heavy alkaline earths anomaly on the basis of classical logic. You therefore need to resort to the approach of convention (Scerri), approximation (Friedrichs), and not losing any sleep over the hard cases (Jones). Well done. The same approach applies to the popularity of the La form. I have hope for you. -- Sandbh (talk) 03:32, 15 July 2020 (UTC)
- That's not true. I already addressed the heavy alkaline earths anomaly on the basis of classical logic. I'm only using your approach as sarcasm, to show how it's absolutely impossible to show anything properly with it. But, since you cannot seem to believe something you don't see in front of you, even if I told you "I've explained the s block situation many times here, there's no need for me to explain it again", here is how to address the heavy alkaline earths anomaly on the basis of classical logic.
- Classical logic demands that if two things are being treated differently, there must be justification for why they are treated differently. So here's the difference: the s block fills preemptively. The symmetry break from pure (n+l) is making the s block fraternise with the next n+l value. So instead of:
1s | 2s | 2p 3s | 3p 4s | 3d 4p 5s | 4d 5p 6s | 4f 5d 6p 7s | 5f 6d 7p 8s | ...
- we have this:
1s | 2s 2p | 3s 3p | 4s 3d 4p | 5s 4d 5p | 6s 4f 5d 6p | 7s 5f 6d 7p | 8s ...
- That's why the anomaly: unlike every other block, the s electrons still remain chemically active even after their block is over, and they can use the higher subshells in the same row since the energy gap isn't big. Theoretically the available subshells should be, say for the sixth row: (4f 5d 6s 6p) for Cs and Ba, also for La through Yb, then becoming (5d 6s 6p) for Lu through Hg, then (6s 6p) for Tl through Rn. Of course it doesn't quite work out perfectly because the d, f, and p collapses are out of sync, so not all are used (for some of them though you can force things; Cs and Ba use 4f under pressure). But you see that it is extremely common for extra orbitals to be used; that idea is fine even if not all of them are actually being used. You call Ca, Sr, Ba an anomaly thanks to using 3d, 4d, 5d respectively. But I say: there is nothing different between them and how Li and Be use 2p, Mg uses 3p, Cs uses 5d. It's the same principle.
- I had a little bit of hope for you before this post, but apparently this is too close to reductio ad absurdum for it to work on you. So I see further debate with you is worthless, aside from making everyone else see how much is wrong with your arguments. Double sharp (talk) 04:39, 15 July 2020 (UTC)
@Double sharp: Re, "making everyone else see how much is wrong with your arguments". No doubt there is so much wrong with my arguments that I managed to fool three PhDs and the editor of Foundations of Chemistry, into accepting my article for publication. Of course, I know! They must all be wrong! Meanwhile my earlier article on metalloids has been cited 24 times, and my article on organising the metals and nonmetals has been accessed 1,935 times, and I have a credit for 61 contributions to the Internet Database of Periodic Tables and, within Wikipedia, 2½ bronze stars. Clearly I know and understand nothing about chemistry, the periodic table, and science for that matter. That must be why it is important for you to make "everyone else see how much is wrong with your [my] arguments". Sandbh (talk) 02:54, 16 July 2020 (UTC)
What chemists need not lose sleep over (but should bear in mind)
- Arguments for Lu in Group 3
- Support for Lu by two of ten active members of this project
- Hard cases at the boundaries
- H in Group 1, despite the fact it invariably completes its valence shell, in stark contrast to the rest of Group 1
- The fact that rubidium and thallium are, after Zr-Hf, the most closely associated pair of elements in the Earth's crust, despite Rb being in Group 1 and Th in group 13
- The fact that Fr, an s-block metal, could be expected to have some p-chemistry
- The fact that Ca-Ba show some d-orbital involvement in their chemistry although they are s-block metals
- The fact that, in some respects, Be-Mg fit better over Zn
- Silver being counted as transition metal even thought it has predominately main-group chemistry
- The fact that gold shows some halogen-like behaviour despite being in group 11
- Group 12 being in the d-block although they have predominately main-group chemistry
- The anomalies going across Ga-Br, and Tl to At
- The fact that, in some respects, Al fits better over Sc (as Pauling did)
- Helium in group 18, even though it has no p-electrons
- The fact that neon, rather than helium, is thought to be the least reactive noble gas
- The fact that argon (Z = 18) has a higher atomic weight than its successor potassium (Z = 19)
- Thorium being in the f-block while not having an f-electron
- The d-block becoming split in the 32-column form given this is not seen in the 18-column form
- First row elements (most commonly in the s-, p- and d- blocks) having noticeably different chemistry to second row elements
- Lr being in the f-block even though it has a p electron
- The fact that the early actinides behave more like transition metals than the rest of the actinides
- Low-lying 4f levels in La may influence some of its properties
- Arguments for Lu based on symmetry considerations: "If one seeks for the maximum chemical utility…[one] should opt for the more 'unruly' tables."
- The fact that chemistry has all sorts of fuzzy definitions
- Disputes arising therefrom
- The fact that some metals have higher ionization energies than some non-metals
- The 5d metals becoming less homogenous with La under Y. Rather than losing any sleep about this, chemists know this is due to the inter-positioning of the Ln between La and Hf, in accordance with the aufbau process
- The fact that the PT used within IUPAC has a 15-element wide "f-block".
I shall stop there as 28 is a perfect number. --- Sandbh (talk) 13:15, 15 July 2020 (UTC)
Crankiness, grumpiness, jibes, and the like
I try to not take crankiness, grumpiness, jibes, and the like too seriously. Sometimes I find these are quite funny, in a good way, for their creativity. The group 3 question has been around for about 60+ years, without resolution. So yes, it is a wicked problem that takes a relatively enormous amount of effort. This can result in occasional and understandable lapses of irritability, impatience, exasperation, and the like.
I ask for patience as those of us who still have an interest in the matter continue to try and unpack the group 3 conundrum.
Itinerant to localised; superconductivity
The context for this post is Double sharp's concern that I did not address the findings of Herbst (1992) re a transition from localized to itinerant 4f magnetic moments, as La is subject to pressure. In what follows I have [numbered] the key observations.
Among other things Herbst also reported that:
- "…[the] 4f bands…are centered 2-3 eV for both dhcp and fcc La above…[the Fermi level] under normal conditions and which rise as the volume is reduced."
- "The 2-3 eV separation between the 4f bands and [the Fermi level] in the one-electron calculations is great enough to limit the 4f states to at best a very minor role in the behavior of the superconducting transition temperature of lanthanum…" (p. 6667)
I draw your attention to Herbst's description of the role of the 4f states in La as playing "at best" a "very minor" role in the behaviour of La's superconducting transition temperature…". [1]
My first reference is:
- Pickett WE, Freeman AJ, Koelling, DD. 1980. f-electron bonding, electronic structure and phase transitions in lanthanum and cerium. Physica B+C. 102, 1–3, 341-346, doi:10.1016/0378-4363(80)90188-6
In this article the authors note that an itinerant to localised 4f change occurs in fcc La (which is stable at 310 °C).
The 4f occupation increases slightly with pressure from 0.05 to 0.10 under a 25% decrease in atomic volume.
This change in 4f occupation is referred to by the authors as "negligible". [2]
At ambient pressure, the more stable dhcp phase of La coexists with the unstable fcc phase.
The authors speculate that the dramatic difference in the critical temperature of superconductivity between La, and Sc, and Y, is largely due to the softer phonon spectrum of La, which seems to arise from "virtual" [?] f-like polarisation related to the unoccupied 4f bands above the Fermi level, in fcc-La.
They say, "Clearly, more experimental work to determine the phase changes in La under pressure would be desirable." However their article has only 13 citations none of which address this. I will return to this question.
On a related note, if thorium behaved as a d-transition element, it would be expected to be an hcp crystal structure metal, as is the case for Ti, Zr and Hf. Instead, it has been shown that the fcc structure in thorium is due to the presence of occupied itinerant 5f states. [3] See:
- Börje Johansson & Sa Li (2009) Itinerant f-electron elements, Philosophical Magazine, 89:22-24, 1793-1799 (1796), doi:10.1080/14786430902917632
Thus for the actinides there are five elements (Th-Pu) showing itinerant 5f behaviour, before localization sets in when the atomic number is increased from Pu to Am, while for the lanthanides only one element exhibits 4f itinerant properties, i.e. cerium, and thereafter localization is energetically favoured for all the heavier 4f elements
The following relationship between the light Ln and the An the arises: [4]
Ce Pr Nd Pm Sm Eu Gd Th Pa U Np Pu Am Cm Bk Cf Es Fm
My reference for this relationship is:
- Johansson B & Skriver HL 1997, "Itinerant f-Electron systems", in DF McMorrow, J Jensen, HM Rønnow (eds), Magnetism in metals: A symposium in memory of Allan Mackintosh, Copenhagen, 26-29 August 1996: Invited review papers, Kgl. Danske Videnskabernes Selskab, Copenhagen, pp. 185-206 (189), https://www.fys.ku.dk/~jjensen/Book/johansso.pdf
Harking back to superconductivity in La, my reference is:
- Bağcı, S, Tütüncü, HM , Duman S, Srivastava GP 2010, "Phonons and superconductivity in fcc and dhcp lanthanum", Phys. Rev. B 81, 144507
The authors write:
- "Superconducting tunneling experiments on La showed that this element is d-type superconductor, with electron-phonon coupling constant around 0.8–0.9. On the theoretical side, previous band structure investigations for La indicated that the 4f electronic states lie a few electron volts above the Fermi level even up to P = 120 kbar and do not play a direct role in determining the physical properties and phonon spectrum of this material." [5]
Conclusions
- There is no support for 4f presence La playing a role in its superconductivity [1], [5]
- The 4f electron states in La do not play a direct role in determining its physical properties [2], [5]
- 5f presence in Th metal plays a significant role [3]
- As far as itinerant f electrons are concerned, and their direct impact, the relationship between the Ln and An starts with Ce and Th [4]
- As far as localized f electrons are concerned, the relationship between the Ln and An starts with Pr and Am [4].
Per [1]–[5], I've consistently argued there is no requirement to invoke f electrons in order to explain the properties of La.
Observation
Double sharp's attempts to somehow corral La into the f-block, on the basis of an f-electron presence, have consistently struck me as a desparate, enormous over-reach, equivalent to trying to use minutiae to leverage the world. I say this with no disrespect to Double sharp the person. Sandbh (talk) 03:19, 17 July 2020 (UTC)
Group 3: Personal opinion v NPOV
This is what is happening here.
I like to think that User:Double sharp and I, while we strongly disagree, base our views of the group 3 situation on a thorough and objective consideration of the literature, to the best of our abilities.
Most of the rest of the editors who have voiced their opinions for Lu, it seems to me, do so on the basis of a personal preference or a very limited grasp of the full extent of the arguments and literature involved in this case. That isn't necessarily a bad thing since these perspectives are not necessarily blinkered by a professional background in chemistry.
I exclude User:R8R from this generalisation since he always, it seems to me, tries to give a balanced assessment.
I'm not holier than thee. I do claim, along with Double sharp, to have maintained a depth of familiarity and interest in this question since at least 2016. In my case I've been arguing the question with Scerri, on and off, since 2008. And along the way, Double sharp and I have changed our minds about Group 3 as we both learnt new things along the way.
Unfortunately, much as it bugs me, I'll have to continue to deal with personal opinions and their associated perspectives. Much as I very much like Wikipedia, and being a part of our Project, that is one limitation of the consensus process. I understand the more I vent about this, the more risk there is of burning bridges along the way. If that is the price of good science, so be it. I'd rather try to manage the risk than cut off my nose to spite my face. I'll rely on my publications in the peer-review professional literature—with due acknowledgement to colleagues here for making me think hard and obliging me to better set out and support my arguments—to speak for themselves. Sandbh (talk) 05:34, 17 July 2020 (UTC)
Draft RFC on Group 3
@Double sharp: I've looked at your spectacular 72,700 word draft (~100 pp, A4) supporting statement for the proposed group 3 rfc. I enjoyed reading it until I ran out of puff after the first 20 or so thousand words. I note our joint submission to IUAPC ran to only 19,000+ words.
I see bias; errors; selective quotations; and out of context extracts of what I said. Now, I don't mind tethchiness within our project, so much. However, since the rfc is going "out to the world" I object to your failure to assume the good faith of my contributions.
Within reason, you can write whatever you like on your own pages. That said, I note User:Dreigorich has provide you with "helpful" feedback. @Dreigorich: per WP:CIVIL I can hardly believe you said, "Well, the draft looks good so far."
I note the draft follows other unacceptable behaviour on your part, including your hack work on our periodic table article; removing some of my citation supported content; slandering me; swearing; and effectively demanding I provide a falsifiable hypothesis when I was under no obligation to do so.
I have half a mind to let the rfc hang by its own rope. That said you've patiently engaged with on the group 3 matter for as long as the thread has had its current duration, this time 'round.
So I suggest you try for another draft, if you want to your rfc to be treated seriously. This is more of a style issue than a content issue. Sandbh (talk) 07:37, 17 July 2020 (UTC)
A "relevant" argument, unfortunately incomplete in sources
Here. Sandbh (talk) 03:30, 19 July 2020 (UTC)
The group 3 RFC
Has begun. Double sharp (talk) 10:10, 20 July 2020 (UTC)
Group 3: Collected thoughts
This section gathers together previous disparate threads to do with Group 3 and related matters. Sandbh (talk) 01:49, 25 July 2020 (UTC)
Article edits re group 3
Officer781 (talk · contribs) has GF edited [2] Group_3_element, and [3] Template:Periodic_table_(group_3)introducing a "6-member" group 3 (include Lu, Lr, La, Ac). Of course this is not 'wrong'. Now I wonder if this is a good presentation altogether, and is this an outcome of the recent discussiuon (both here at WP and in RL by IUPAC).
I remember years ago we decided to present one form as prevailing, and have the other form well described in detailing articles (dedicated sections). Now the presentation seems to become a combination of two forms, if I understand this right. Or maybe: the forked form? Anyway, what do we want to present (at which detail level)? Whatever we do, should be consistent over all PT articles. -DePiep (talk) 17:56, 6 June 2020 (UTC)
- @DePiep: I guess the idea is for a neutral presentation until we make up our mind? I would prefer if it somehow showed for the sixth and seventh row "either La or Lu" and "either Ac or Lr", to make it clear that the "bifurcation" option is not actually a common one and is not among the ones IUPAC is considering.
- (Of course, by now you know that what I would strongly prefer is default Sc-Y-Lu with a note saying that Sc-Y-La is also common. And then a large section on this very issue based on reliable sources. Which I note tend to support Sc-Y-Lu when they focus on the issue. ^_^) Double sharp (talk) 04:45, 7 June 2020 (UTC)
- @Officer781, Sandb, and Double sharp:. OK. That is, it is an "or" presentation, not a six-member group (which would be incorrect, confusing and hard to clarify if we present it like that). Excludes the forking from. "Group 3 is either Sc-Y-La-Ac or Sc-Y-Lu-Lr". Could be temporal, since WT:ELEM or IUPAC/Scerri could result in a preferred form. Shall we formally propose that here, for consistency over all PT articles? -DePiep (talk) 09:43, 7 June 2020 (UTC)
- (fix ping @Sandbh: -DePiep (talk) 09:45, 7 June 2020 (UTC)
- @DePiep: I do not like it for the same reason I would not like calling the Al article "alumin(i)um". It makes the controversy into a big deal that appears everywhere even when it is irrelevant. I would instead strongly support Sc-Y-Lu throughout as the default (i.e. rollback to where we were before the previous RFC), as supported by the majority of reliable sources focusing on it, supported by a majority in the straw poll above, and supported by the scientific arguments already given above by Droog Andrey and myself. Sc-Y-La can be mentioned as an alternate form in the same vein as how we mention Sc-Y-Lu. That is, our flagship periodic tables would change to look like they do at User:Double sharp/Sc-Y-Lu, and we would simply discuss the group 3 question where it is relevant. Double sharp (talk) 10:00, 7 June 2020 (UTC)
- (fix ping @Sandbh: -DePiep (talk) 09:45, 7 June 2020 (UTC)
- OK, no (intermediate) two-valued main show then (I agree with the no-big-deal argument; a bit similar with H-positioning and metalloid-members). But for that change you prefer, we need a firm conclusion in the big discussion. How is the process going? Is such a consensus building (beyond !vote counting)? Will an RfC or similar be started? (no cynicism intended). Meanwhile, what do we do with the edits Officer781 did, see OP? We could revert those for being 'GF but no consenses'. -DePiep (talk) 10:16, 7 June 2020 (UTC)
- The consensus here seems reasonably strong, but since the previous change involved an RFC, so will this one. I plan to start that in a month when I have the time for it. As for Officer781's edits: I personally do not object to them as an interim measure. I don't know what the others think. Double sharp (talk) 11:40, 7 June 2020 (UTC)
- OK, no (intermediate) two-valued main show then (I agree with the no-big-deal argument; a bit similar with H-positioning and metalloid-members). But for that change you prefer, we need a firm conclusion in the big discussion. How is the process going? Is such a consensus building (beyond !vote counting)? Will an RfC or similar be started? (no cynicism intended). Meanwhile, what do we do with the edits Officer781 did, see OP? We could revert those for being 'GF but no consenses'. -DePiep (talk) 10:16, 7 June 2020 (UTC)
- Just received the pings. I did not mean that it is a 6-member group, but a 4-member group that is ambiguous. I feel that Wikipedia should not "choose a prevailing form" because that implies a bias on the part of Wikipedia. Since Group 3 element is an article that goes into the depth of group 3, I believe the template and the article is appropriate to discuss the nuances of the issue especially since the text already includes both possibilities.--Officer781 (talk) 12:58, 7 June 2020 (UTC)
- @Officer781: My problem with not choosing a prevailing form is basically that every time you display a periodic table you either have to pick one form or show the ambiguity with a footnote. If you do the latter, you're not only reflecting no sources (because AFAIK almost everybody just picks one form), but also often engaging in an irrelevant tangent for the article the table is drawn on (how much does group 3 matter for neon?). So, maybe having the group 3 template in particular look like that makes sense, but I think the general periodic table we draw on most articles as a navigation aid basically has to pick one side or the other. Which may as well be the side the relevant sources generally take. ^_^ Double sharp (talk) 13:39, 7 June 2020 (UTC)
- re Officer781: yes, the Group 3 article should reflect the current situation. So presenting the group as "form one or form two" is OK, and an extensive, sourced description of both is fine. This is what I call a "dedicated article" or "dedicated section".
- But. This 'or'-situation is not viable in more generic articles about the PT. It would not help to enter both group 3-forms in the Periodic table#Overview. The same issue exists with positioning of H, and with exact borders of metalloids-category (include Po?). Such issues have a preferred presentation form (mainstream in science, not a WP/OR choice BTW), and are described & detailed in their dedicated articles§ions, all right. Now once an RfC or IUPAC has decided on a scientifically preferred group 3 constitution, we will use that one for our general PT's (e.g., in the neon article). At this moment enwiki uses a decision that the preferred general form is Sc-Y-La-Ac, and so we keep that in the generic PT's. Meanwhile, improving Group 3 element to reflect the or-situation in detail is welcome. But not the generic articles then. HTH. -DePiep (talk) 18:01, 7 June 2020 (UTC)
- Just received the pings. I did not mean that it is a 6-member group, but a 4-member group that is ambiguous. I feel that Wikipedia should not "choose a prevailing form" because that implies a bias on the part of Wikipedia. Since Group 3 element is an article that goes into the depth of group 3, I believe the template and the article is appropriate to discuss the nuances of the issue especially since the text already includes both possibilities.--Officer781 (talk) 12:58, 7 June 2020 (UTC)
Useful reading
Grochala: doi:10.1039/C7CP03101G. Notice the analogies of Yb to Hg on p.10. Double sharp (talk) 07:50, 18 June 2020 (UTC)
- Yes, that inspired my Ziggurat. Sandbh (talk) 02:22, 27 July 2020 (UTC)
The location and constitution of Group 3 of the periodic table -- FoC status
I received the proofs of my FoC article today. I have two days to check and correct them. Based on past experience, the article will appear online shortly thereafter. Sandbh (talk) 23:01, 19 July 2020 (UTC)
- Sigh. I had made changes to the MS in response to feedback from the editor. These changes were arbitrarily removed by the outsourced (to India) production company as they did not meet the journal style guide, which is not available on line. I rejected the proof and asked for another one, with my changes reinstated. I am co-authoring another article for a different publisher, based in Switzerland, and the difference in the way they handle an MS is extraordinary; nothing is too much trouble. Outsourcing kills customer service and accountability. Sandbh (talk) 02:08, 24 July 2020 (UTC)
Post-mortem retrospective
OK, a few days later I think I can say this dispassionately to Sandbh.
Two places. I strongly suspect that the main reason the megathread ended up raging in circles this way is simply because of one key point: La arguments and Lu arguments start from two very different places. La arguments seem to value DEs and ground state gas phase configurations, Lu arguments do not value either at all. Lu arguments consider the question of 4f character in La and Lu to be something extremely important for deciding the issue; I suppose for La advocates this is probably not so important. Faced with starting from different premises, it is no surprise that neither side manages to convince the other very well. The Lu side finds it hard to believe that 4f character in La could be called unimportant, the La side finds it hard to believe that DEs could be called unimportant. Let's not argue who's right, it will start the whole thing again.
Now. I believe I have calmed down sufficiently to be able to offer a little bit of criticism without doing it in an angry tone. So, please take this as a summary of why I feel we are still in disagreement, albeit a gentlemen's disagreement now.
SSC. To my taste, you apply "simplest sufficient complexity" too readily. When there is a phenomenon, you want to apply the simplest possible explanation. That is in itself not bad. But then you stop there just when I feel it would enhance understanding to go deeper.
The reason why I do not feel comfortable with this approach is that science seems to only apply "simplest sufficient complexity" in some ways. In the Occam's razor sense, absolutely! That's why geocentrism lost to heliocentrism. But they do not seem to apply it to stop at the first level. Instead they do keep drilling down. That's just reductionism. When Priestley first discovered the law of definite proportions, it was interesting. Then Dalton put it on a firm foundation with his theory of atoms. But we didn't stop there; instead we went further, drilling down past the atom into the electrons, into the subshells, into the molecular orbitals that result from chemical bonding, and a whole exciting branch of chemistry was born.
Discussing Lr. The other reason is that I think sometimes it does not help your understanding. Well, let me take an example from Archive 42, since that was the one where the discussion was most civil. You listed some properties where group 3 seems to be different from group 4, you argued that it supports a group divide, and I argued that this is simply the oxidation state difference. Well, you said it passed simplest sufficient complexity. However, an example where I think (respectfully) that it hurt your understanding is when you discussed lawrencium a few days ago with Dreigorich. You said:
| “ | The volatility of LrCl3 is similar to that of the chlorides of Cm, Fm and No, and much less than that of Rf. | ” |
But this is not comparing like with like. The chlorides of the actinides you mention are CmCl3, FmCl3 or FmCl2, NoCl3 or NoCl2. The chloride of rutherfordium is RfCl4. The oxidation states are different. And the reason why this is problematic is because the oxidation state impacts volatility, which is what I mentioned. The higher the cation's oxidation state, the less ionic and more volatile the compound is going to be. Again, this is why I feel Restrepo's article is not definitive: if you only consider stoichiometry, similarities between elements in different oxidation states cannot be noticed.
We can drill down another level and explain it almost from the basic concepts. I know you are likely to consider it to not be simplest sufficient complexity. But please, allow me to do it in one paragraph as an illustration. Well, in a higher oxidation state, the ion is more polarising by Fajans' rules, there is less chance for a real ionic bond to occur. So instead you are more likely to get a covalently bound molecule. That is also why the counterion matters: for chlorides maybe the difference is from valence 3 to valence 4 in this part of the table, but for fluorides it tends to be valence 4 to valence 5. Except in the first row due to small sizes, then it's even smaller even for fluorides: LiF is ionic, but BeF2 already has a lower melting point and has significant covalent character. Finally BF3 and CF4 are low-melting molecular gases.
Now, you can say that this isn't simplest sufficient complexity. But the issue is that it is the sort of explanation of periodicity that you will already get in high schools. See Chemguide for example for the period 3 chlorides. This sort of drilling down is absolutely standard when it comes to teaching students. You can see that Jim Clark is using what is exactly equivalent to my explanation above. Okay, he uses electronegativity, I use polarising power. Evidently the UK syllabus prefers to describe bond polarity using Lewis and Pauling's idea (start from a covalent bond and polarise it), I prefer Fajans' idea (start from an ionic bond and polarise it), but there isn't a real difference. (That's just pedagogy; I think Fajans is easier to start with just because it is fairly easy to understand what is going on with ionic bonding, whereas with covalent bonds you need to make the point that electrons are distinguishable and the atoms cannot distinguish whose electrons are whose.) Actually Clark also discusses Fajans' idea on his page on electronegativity anyway!
So, back to the period 3 chlorides. Clark notes MgCl2 melts at a lower temperature than NaCl and points out that a pure ionic bond model would predict the opposite – for reasons that he himself drills down into (higher charge should give greater attention). So he explains it as a result of Mg being less electropositive than Na.
And then he goes on to explain the rest of period 3 in these terms. AlCl3 is not a really ionic compound, he attributes that to electronegativity. SiCl4 is definitely covalent, he also attributes that to electronegativity.
Why do I mention this much about Jim Clark? Because his site is trying to follow UK high school syllabi. Notice that he never mentions anything about group divides for 3 vs 4, or the almost identical case of 13 vs 14 (same issue, aqueous cations exist for group 13 metals but not group 14, everything coming from rgw +3/+4 oxidation state difference still holds). None of that. Only statements about electronegativity and trends. That implies that in those UK high school exams, if you are asked why AlCl3 melts higher than SiCl4, you are not expected to say anything about group divides. And that you will probably not get any marks for saying it. Electronegativity differences, however, will surely get you your marks.
At this point one can look at all those transition metal halides and see that this approach works and the group-divide one doesn't really. ScCl3 is indeed ionic where TiCl4 is covalent, but: TiCl3 is ionic, TiCl2 is ionic. And we know that elements become more electronegative in higher oxidation states (most obviously for Tl and Pb, but even here it is significant). Maybe this is not so common to discuss in UK high schools, but you can see what Droog Andrey teaches his students in his capacity as a chemistry instructor at the Lyceum of Belarusian State University: "Оксиды и галогениды металлов часто имеют ионную природу (MgO, FeCl2, AlF3), но в случае низкой активности либо высокой валентности металла образование катионов этого металла может стать невыгодным (например, вещества HgCl2, OsO4, WF6 построены не из ионов, а из молекул)." – he points out the generalisation that when the valence is high or the metal activity is low, forming cations becomes unprofitable (HgCl2, OsO4, WF6). It must be standard for the Belarusian university syllabus, at least. And although that's maybe not in the UK syllabus, in principle everything you need to work it out is there, because Clark discusses lattice energies and Born–Haber cycles on Chemguide! (Just examine the ionisation energies of Hg, or what is needed to get Os8+ or W6+, you will see it.)
Stopping early. So, I put it this way: if you want to stop early, say you have reached "simplest sufficient complexity", that is your choice and your right. But I think you should be aware that chemists round the world appear to disagree. And they seem to disagree enough that they engage in what you refer to as drill-down complexity in the syllabi that they set for their students and expect the students to reproduce that drill-down complexity to get marks.
Descriptivism vs "predictivism"
So that's the first point. The second one is about descriptivism vs "predictivism".
If you just want to look at the properties and classify them in a way that seems to make sense, the current table with He-Ne and Sc-Y-La is not so bad. It works fairly well at keeping similar elements together. Maybe lanthanum is a bit oddly reactive for a transition metal, but OK, maybe we can argue that iron forms a spalling oxide too. Where it looks the worst is lawrencium, whose emerging chemistry is definitely not like the late actinides but is quite like the early 6d metals rutherfordium, dubnium, and seaborgium. But we can sweep that under the rug as one short-living anomaly if you want descriptivism, because lawrencium's longest-lived isotope has an eleven-hour half-life, you get one atom of it at a time, so nobody cares.
The only trouble is: a table with He-Ne and Sc-Y-Lu is also not bad. (Maybe even better. Lu works OK either way, it is similar to heavy rare earths and early third-row transition metals. But La and Ac are with distinctively more similar elements this way, and most of all Lr finally looks like it is in the right place.) The Pauling-style table with aluminium over scandium is also not bad. (Again, maybe even better for aluminium.) So is the table with beryllium and magnesium over zinc. (Again, maybe even better for magnesium.) And, until transuraniums were discovered, a table stopping at U and putting Th-Pa-U under Hf-Ta-W would actually look even better than a table stopping at U and putting Th-Pa-U under Ce-Pr-Nd. The fact that they all look very good at describing the properties of the elements shows that this will not give a definitive answer: the only way in which He-Ne and Sc-Y-La is distinguished is that this happens to be the most common form at present. If we discussed this around the 1940s then a table with He-Ne, Sc-Y-La, but also maybe Be-Mg-Zn, and definitely also Th-Pa-U under Hf-Ta-W, would be also not bad and would have the advantage of incumbency. Maybe that's OK in a "don't lose sleep about it" kind of way, but I'm sorry to say that I do not think that is in itself a good justification for the current table, if you want to examine which table's better, when it would have supported any of the reasonable tables I mentioned if they were currently the standards. The last one (Th-Pa-U under Hf-Ta-W) even was.
And: the fact that Be-Mg-Zn was eventually replaced by Be-Mg-Ca, the fact that Th-Pa-U were moved to the actinide series, shows that indeed chemists do lose sleep about such things. Maybe they are conservative, but eventually when the evidence became overwhelming and impossible to ignore for the average chemist, the change got made. Indeed the properties of lawrencium are so bad a fit with late actinides, compared to their good fit with early 6d metals, that I almost suspect that had the strong force been a little bit stronger, so that lawrencium was at least quasi-stable and available in bulk for all chemists rather than just a few lucky ones, the Lu form would've long since triumphed. But OK, enough of hypotheticals.
So what to do? I think that many would not want to just take the properties and classify them: no one would be able to remember all of that. At least I want to understand them. That way allows one to not only organise the chemistry of the elements with a periodic table, but also go the other way: with a periodic table based heavily on drilling down to the lowest level of theory, you can predict extremely well properties of the elements that you don't already know. You can see the regularities, connect them back to the basics, and even get out all the course corrections that will make your prediction almost right on the mark.
Let me give an example. Rhodium, for instance. Well, with my approach I very proudly drill down to the chemically active subshells: I read off [Kr] (4d 5s 5p)9 from my table, and get that [Kr] 4d7 5s2 5p0 should be a configuration within energy range of chemical activity. Because of the radial nodes of 4d that 3d doesn't have, I expect rhodium to be happier in higher oxidation states than +2 and use the d electrons; but I also know that 4d drowns quicker near the end, therefore I suspect only fluorine will give the really high oxidation states. So I expect oxidation state +3 or +4 to be the most stable. Of course, this is a transition metal, in general I would guess that everything from +2 to +6 should exist, the latter mostly just in a volatile hexafluoride. This is a late 4d element, the electronegativity should rise, the class-B-ness should also rise, and therefore I suspect that the aqueous chemistry of rhodium will not heavily involve aquated cations. Finally, interpolation from cobalt, ruthenium, palladium, iridium suggests we will have a very noble metal indeed.
That is the sort of power I want to get out of the periodic table: not just to describe, but also to predict. If you imagine what it is like to value that, then maybe you will understand why I so greatly dislike saying that some things in the periodic system only apply to their context. If I am trying to use the periodic table to predict properties of an element I've forgotten everything about, or that nobody knows anything much about in the real world, then how do I know which context is supposed to apply?
And it is not just me. That is why Mendeleev was remembered and the other pioneers of the periodic law were not. Because he dared to predict properties of his unknown elements. (Re the last sentence: note that the most famous of Mendeleev's gaps, Sc, Ga, Ge, and Tc, were almost completely surrounded by known elements. Scandium on all four sides, the other three on all but one.) Maybe Mendeleev did not believe in atoms, much less electrons. But "money talks", speaking figuratively. If he had our current information, but somehow not yet the periodic law, he probably would've believed in them. And I am quite sure that if he had tools to make even better predictions, he would've leapt at the chance.
That, essentially, is why I favour He-Be and the Lu table. It lets you get more out of your periodic table because of its consistency with the general trends. All the extra complexity and drilling down is widely regarded as well worth it to get the ability to predict chemistry of elements you know nothing about, and I believe that for general chemistry purposes this ability is very important. Not only do high school teachers want the extra complexity and expect you to state in in the exam, but that kind of prediction is exactly what Mendeleev did. And it will be what chemists try to do when you ask them about how one of those superheavies that basically nobody knows anything about:
| “ | Hassium. I know nothing about hassium. Should we make something up?
Element 108 – hassium – I know absolutely nothing about it. However, the power of the periodic table is that by looking at the periodic table I can say: well, its chemistry has got to be a bit like iron, but it won't be exactly like iron because iron is a light element, and as the elements get heavier going down, that their chemistry changes. They tend to have a larger number of so-called oxidation states. And so, what I would predict is that if hassium was not radioactive it would still be pretty poisonous, and it might be an interesting catalyst. It would probably react with carbon monoxide and if it were stable it would have made a great addition to my doctoral thesis work. |
” |
| — Martyn Poliakoff, Periodic Videos, Hassium (1st version) | ||
Contexts. If one says that there are some arguments that can only be applied in their contexts, not the whole table, Prof. Poliakoff could not have done this. How would he have known which context hassium would be in?
That's why I claim that indeed, the La table is "less than useful" for predictive purposes, to refer to the quote you often post. And, again: when chemists use the periodic table, predictive purposes seem to be more important.
Of course, the trends He-Ne, Sc-Y-La, B-Al-Sc, C-Si-Ti, Be-Mg-Zn etc. are worth looking at. I do not mind adding them as secondary periodicity even on my preferred form of the table. I simply claim that one gets more predictive power from the periodic table if it is He-Be and Sc-Y-Lu. It is not about regularity and symmetry, but about predictive power. That is why I do not consider it a problem, for example that the first row is the only one whose length is not repeated, because one can get predictive power from that symmetry break. Well, you know the drill, 1s has no shielding at all from the nucleus and is already small to begin with by kainosymmetry, the result is obvious to everybody: it should have the greatest first-row anomaly of all as both delayed and immediate effects of kainosymmetry occur together. That's why for me helium goes over beryllium. Above neon it is not severely anomalous, above beryllium it is. And it is a powerful statement that even hydrogen belongs as part of the periodic law, and that even that weirdness is part of the generality.
All that said, this is not an attempt to convince you. Just to explain what I'm up to, and what the chemistry literature is up to. If you are fine with your approach, that's fine. I am not your teacher, and you are not going to be docked any points for disagreeing with me. ^_^ It simply means to highlight one point that I think impacts why we choose differently. I choose a periodic table that is most suited to what I want out of the system, and presumably so do you. I just want my periodic system to give me a lot. And, judging from the comments quoted above: so do chemists.
No good case for change on Wikipedia. I recognise that currently there is not a good case for changing it here. The problem is that while the generalisations that I mention are standard stuff in the literature, and some thought reveals that the Lu form is a natural consequence of taking them seriously as a basis, the Lu form itself is not. And if you look only at articles focusing on the placement of helium, they will obviously skew towards supporting He over Be because most He over Ne advocates think that placement is too obvious to need defending, so arguing for looking at such sources does not quite work when it comes to Wikipedia. So we can say standard stuff that so happens to, if you think about it, support Lu. But we cannot say that it supports Lu unless the source says so, and we still cannot use the Lu form (except to illustrate the argument) until most people do that.
However I continue to believe He-Be and Sc-Y-Lu is the ideal rectangular-grid table for students and will continue to use it when WP policy is not at stake. Such as the one I show on my userpage. Well, maybe just Sc-Y-Lu for first-year students. He-Be can wait until we're sure that they will not try to suggest HeO as a room-temperature stable compound. ;)
Eventually we will, I suppose, follow IUPAC on Wikipedia when it comes to a decision. And I strongly hope their decision is Lu. But if they decide on La, I will accept the continued use of the La form on Wikipedia (though I will suspect that eventually the calls will be enough to make them reexamine it again in that case), even though I will likely persist anyway in using the Lu form outside it. (IUPAC approval doesn't dissuade me from He-Be, after all. Just from using it here. ^_^)
And for the rest of you: if you were convinced by all this to the Lu form, you have my thumbs up. If you were convinced by it to the La form, I guess you have Sandbh's thumbs up. And if you are overwhelmed and cannot decide, well, the IUPAC project is still making up its mind. I don't think we're going to argue this much again until and unless someone has a significantly new idea thrown in, so you have all the arguments already. Double sharp (talk) 09:48, 23 July 2020 (UTC)
@Double sharp: I saw your post-mortem yesterday, before you placed a moratorium on it.
I liked the way you approached the question as a difference in philosophies. That is how I saw it originally: La as an Aufbau-chemistry form plus symmetry breaking; Lu as a philosophical idealised form per Jensen-Scerri, along with a hefty dose of regularity and symmetry.
I liked your descriptivism v predictivism approach. I think both forms are fine for either approach. It depends on the context. Along with Maw-Kuen Wu, Paul Chu discovered the first superconductor above liquid-nitrogen temperature. They had observed superconductivity in metastable LaBa2Cu307 (LBCO) and that their data suggested a smaller trivalent element than La should alleviate the instability impasse. In late January 1987, his group at the University of Houston and the group led by his former student Maw-Kuen Wu at the University of Alabama observed superconductivity at 93 K in the stable compound YBa2Cu307 (YBCO).
When I walk into the Periodic Table bakery now, I see a rich smorgasbord of periodic pastries. The La pastry is a best seller. That does not mean that the *-**- and Lu pastries have been forgotten. Palates and dining purposes (breakfast, morning tea, lunch, afternoon tea, dinner) vary. Sandbh (talk) 03:06, 24 July 2020 (UTC)
- @Sandbh: OK, I have restored the post-mortem since it seems to be a productive discussion now. If you feel it stops being productive, please say so and we'll stop it again.
- Regarding what you said about superconductors: please note that lutetium was also tested along with yttrium. And the Lu analogue is also superconductive (p. 42) with critical temperature around 90-100 K also. So, maybe this is not totally conclusive: the Lu table seems to be just as fine at the La table at predicting this one.
- Regarding the best-selling status of the La pastry. I hope you do not mind if I respectfully dispute this. The IUPAC survey breaks things down by decades, and you can see that the La form is steadily losing its majority since the 1990s:
| Decade | La under Y | Lu under Y | * under Y | Total |
|---|---|---|---|---|
| 1970s | 18 69% |
2 8% |
6 23% |
26 |
| 1980s | 31 78% |
6 15% |
3 8% |
40 |
| 1990s | 27 82% |
2 6% |
4 12% |
33 |
| 2000s | 38 62% |
14 23% |
9 15% |
61 |
| 2010s | 16 48% |
6 18% |
11 33% |
33 |
- And in fact in the 2010s it has only a plurality, with * going strong. I think this supports the idea that La should not be treated as a "default" anymore. How do you see this? Double sharp (talk) 03:34, 24 July 2020 (UTC)
@Double sharp: On LrCl3, Dreigorich said:
- "Lr is so different from Es-No that it doesn't make sense to put them together. Lr is more like Rf-Hs than it is to Es-No."
To which I responded:
- "Lr is not so different from Es-No. The most stable oxidation state for Es, Fm, Md and Lr is +3; No is the exception, being +2 (it still shows +3 too, however). The volatility of LrCl3 is similar to that of the chlorides of Cm, Fm and No, and much less than that of Rf."
Considerations of like-with-like didn't enter into the picture for me. I only wanted to illustrate that what Dreigorich wrote did not ring true.
He over Be has never bothered me much. Schwarz, OTOH, says this requires, as a chemist, much humour. It's just a question of explaining the context to readers, colleagues, and students for whatever PT is under consideration. An Lu table is fine from that perspective.
I think that addresses your context question.
What you get from a periodic system or table is what you put into it:
- The so-called IUPAC table is more of chemistry-focused table.
- The form with lutetium in Group 3 is more of an idealised table (Scerri 2020).
- The form with lanthanum in Group 3 is more of a pragmatic "no need to lose sleep" table (Scerri 2020).
- Aluminium over scandium is more of a metallurgist’s table (Habashi 2008).
- In the Earth Scientist's periodic table, group 14 is composed of carbon, silicon, titanium, zirconium, and hafnium rather than the standard set of carbon, silicon, germanium, tin, and lead (Railsback 2003).
- In a solid-state physicist's table, both lanthanum and lutetium, as 5d metals, would go under yttrium (Bosko & Chevary 1993)
- In an astronomer's periodic table, hydrogen and helium would be the only non-metals and all the other elements would be metals (Leach 2006).
- A periodic table with hydrogen over boron makes for a nice designer table (Luchinski & Trifonov 1981)
- In a Pauling electronegativity-focused table, group 3 is boron, aluminium, scandium, yttrium, lanthanum, and actinium (1988).
- In a superconductivity periodic table, group 2 is split into barium and radium; and calcium, strontium, and ytterbium, and group 12 is beryllium, magnesium, zinc, cadmium, and mercury (Wittig 1973).
How do you know which context to apply? Through experience, always keeping an open mind.
If you don't know, start with the one you're most familiar with, and work outwards from there. A better chemist is one who can keep all of the perspectives in their head, at the same time.
As Poliakoff (2011) said:
- "In the end, I think that one should remember that Mendeleev devised the PT for a textbook to help rationalize the mass of facts in inorganic chemistry…For me, the PT remains a tool to help reduce the complexity, not a metaphysical truth that has a correct form yet to be discovered."
- Poliakoff, M.: In Bradley, D.: "Periodic debate: Complete not finished." ChemViews Magazine, 9 June, https://www.chemistryviews.org/details/webinar/1077259/Periodic_Debate.html?page=24 (2011) viewed 4 Jan 2020
The learning is to consider how many insights and how much understanding could be gained from appreciating these different stepping stones including, but not limited to the fundamental and important nature of inanimate matter (Valery Tsimmerman, pers. comm., 17 Jul 2020).
Scerri reckons:
- "On chemical grounds the left-step periodic table may seem to be inconsistent and unnecessary since it suggests a chemical kinship between helium and the traditional alkaline earth elements. Although this objection appears to have been partly challenged by the recent observation of a compound of helium with the formula of Na2He, I suspect that the majority of chemists would still not be willing to release helium from its status as a noble gas. This is because the observation of this exotic compound of helium has been carried out at the extremely high pressure of 13 gigapascals.
Yes, on superconductors, it struck me as odd that Sc-Y-La all do it, yet does Lu not(?).
For the survey counts, the 2000s and 2010s combined have the following ratio 2.7: 1: 1 or 57½% to 21¼% to 21¼%. For the *-** count, a significant number of these will be based on a mistaken impression of the status of the IUPAC table. For the Lu figures, a significant number of these will be based on Jensen's arguments, which Scerri & Parsons (2018) described as being too selective. As I see it, the La form retains a meaningfully clear majority.
On simplest sufficient complexity, nothing stops a chemist from drilling down. It is a question of scale. How far should a map maker drill down? Most people are happy with, say, an atlas. If you drill down far enough you will have a map that covers as much area as the land surface of the world. Most people are happy with a map of the broad contours. Hence the popularity of gas phase electron configurations. --- Sandbh (talk) 06:37, 24 July 2020 (UTC)
- @Sandbh: Well, I see your approach. I am still not convinced by it and continue to prefer mine. So let's just leave it at this, which I hope is phrased very gently: we seem to value very different things, have very different readings of the literature, and different ideas about what level of complexity is best, what is more or less scientific, and the relevance of classical logic as opposed to the fuzzy one you seem to favour. And of course we each seem to disagree, sometimes strongly, with what the other party values. For Wikipedia, that's fine. We can work with that on WP when it comes to redrafting the group 3 sections and articles neutrally. But I think in future I will recuse myself from reviewing any future articles that you write for off-WP publication and bring here for peer review, because these differences mean that your ideas are not likely to convince me and my criticisms likely not to convince you, and therefore it will not be a terribly productive activity for either of us. And likewise I do not think it will be terribly productive to debate with you other things such as metallicity classification; we will disagree on what is important in the same way. So, while I thank you for making me think in the past: I intend to instead discuss these things mostly with Droog Andrey in future, as his perspective and understanding of chemistry and its literature is something I agree much more with, and it will be more productive for all concerned. So, no more megathreads here, hopefully. At the most I may simply offer short and to-the-point clarifications to make your definition literally give the result you prefer, like what I did below regarding that antimony cation. But no more than that.
- So, just one final clarification regarding my perspective. Let's not debate it; I don't debate your perspective expressed above anymore, so I'll request that you don't debate what I express below. It is just so that we each know what the other one thinks before leaving this issue until IUPAC's project says something.
- I support the idea that there is one ideal periodic table for all contexts and that it is He-Be + Sc-Y-Lu-Lr for the first seven periods. (So, the Janet table, except that the s block is moved to the front. Just like what I show on my userpage.) Not totally sure about the predicted 8th period as long as no calculations are yet detailed enough, but to my taste Droog Andrey's version (119-120 s block, 121-142 g block, 143-156 f block, 157-166 d block, 167-172 p block) seems the strongest contender. The contexts you mention where different placements are useful are what I simply consider as general secondary relationships that are also valid for all contexts. Like what Droog Andrey drew:
- But I think it is OK if secondary relationships are not explicitly shown, only primary ones. Because the secondary ones can usually be reconstructed off counting columns and following general ideas just like how we read off a lot of trends from the table that are not explicitly there anyway. For me, understanding the generalities and being able to drill down to ground zero is what makes one a better chemist. These secondary periodicities are just one aspect of those generalities for me.
- So, now we both have a dispassionate summary of what the other party thinks and can move on to something more likely to be useful.
- For what Wikipedia shows as its standard periodic table, I've decided not to really care as long as Lu has not the IUPAC project recommendation or the majority of textbooks yet. That's my approach to helium over beryllium, so I may as well adopt it also for lutetium under yttrium for Wikipedia. I guess I would vaguely prefer * for neutrality but honestly, as long as it cannot be Lu, it might as well be either alternative as far as I am concerned. So I will not complain anymore if it stays at La on Wikipedia for now. For what Wikipedia says about the dispute, I only care that the Lu side is portrayed correctly, as I trust Sandbh will make sure of that for the La side. He-Be and Sc-Y-Lu-Lr will stay on my userpage and my unfinished page describing my current view (plus its sort of subpages User:Double sharp/Teaching periodicity and User:Double sharp/Electronegativity), but that's all you'll see of it from me on Wikipedia as long as the situation outside doesn't change seismically in favour of either of them through either the IUPAC project making their recommendation or textbooks converting to them en masse. If and when either happen, of course, I reserve the right to propose them. But that is conditional.
- Right, I think that's it. Sandbh, if there is anything on those subpages or my userpage that you object to in terms of tone or language, let me know and I will change it. Double sharp (talk) 09:59, 24 July 2020 (UTC)
@Double sharp: Please do not offer me, "short and to-the-point clarifications to make your definition literally give the result you prefer, like what I did below regarding that antimony cation" unless I specifically ask for them. That only results in groupthink and a loss of individual creativity, uniqueness and independent thinking. If everybody thinks the same then no one is doing any hard thinking. That is the benefit of a megathread.† Great things can arise and spin-off from a contest of ideas. Sandbh (talk) 12:42, 24 July 2020 (UTC)
- † You may disagree, of course ^_^
- @Sandbh: I phrased this badly. What I meant is, I knew beforehand that you don't consider it useful to put antimony down as a metal, but you gave a definition that literally includes it. That's why I offered the short clarification pointing that out. That's the sort of thing I meant to say I may still do in future, but probably not much more. I have said why.
- As for megathreads: I think we have gotten all the benefits out of this megathread that will be gotten on this topic. I got a good understanding of exactly what I find important philosophically and chemically, how it differs from what you find important philosophically and chemically, and strongly developed my case for Lu. Hopefully you got something out of this too. I just don't think it'll be that useful to have such a contest on any further chemical topics because we have learnt in this one what I find important seems very different from what you find important. It will simply end up as talking past each other: judging from this past one, I will find I cannot philosophically accept your criticisms and probably vice versa. So, I think it's better if we simply present our ideas separately and not attempt to contest each other directly. That way we can put the ugly side of this behind us and simply keep the benefits each of us got from it.
- I plan to take a break from this. I'm pretty securely convinced by my above approach, so I can just finish R8R's collaborations and then Wikibreak again. Maybe we will come back to metallicity in the end and secure that one too to my taste, but again: we wait. That seems more complicated than helium extremism plus the eka-yttrium holy war. In good time. Double sharp (talk) 14:12, 24 July 2020 (UTC)
Landau & Ligshitz (1958)
Mark Leach, who runs The Internet Database of Periodic Tables, has kindly uploaded my observations about L&L. Sandbh (talk) 05:31, 24 July 2020 (UTC)
- ffs, Sandbh, if it has to do with #Group 3 then say so. -DePiep (talk) 00:19, 25 July 2020 (UTC)
- It is indeed about Group 3, as casual reading of the link would reveal, which is why it's now in this subsection. Sandbh (talk) 06:47, 25 July 2020 (UTC)
Periodic table article
I've restored the Group 3 content to the 21 Mar 2020 version, noting most pre-restoration group 3 content is now more appropriately located in our Group 3 element article.
I'll next review the restored content for the two main options, La or Lu, at a now appropriate ~300 words apiece, to see if it's still up to date.
After that I'll hazard an exploration into the depths of our Group 3 element article. Sandbh (talk) 07:10, 24 July 2020 (UTC)
- Is this running out of hand? This is not an isolated edit, ffs. It is part of a big & wide discussion, so please do not present it as an incidental bold edit. Jeeee. -DePiep (talk) 20:24, 24 July 2020 (UTC)
- This 07:01, 24 July 2020 edit it is about, I understand. -DePiep (talk) 00:16, 25 July 2020 (UTC)
@DePiep: No, it's not running out of hand. Double sharp and I have discussed previously. A this time, there is nothing substantial needing further discussion about Group 3. There is more work to do, as I set out above.
I note there is some excitement about the template appearing in our element info boxes. I've expressed my opinion about that. Sandbh (talk) 02:41, 25 July 2020 (UTC)
- Very very confusing, especially since in this PT talk the group 3 issue appears again. Best close this thread, would be OK. DePiep (talk) 20:07, 26 July 2020 (UTC)



