Wikipedia talk:WikiProject Chemistry/Archive 32
| This is an archive of past discussions on Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 25 | ← | Archive 30 | Archive 31 | Archive 32 | Archive 33 | Archive 34 | Archive 35 |
Hydride compound article names
I recommend that some of the hydride compound articles be moved, for either one, or both of two reasons: firstly, to specify stoichiometry, and secondly to place emphasis on the status of the hydride as the standard state. For instance, since Diborane is the standard state, saturated boron hydride, it could be moved to Boron(III) hydride. This name is suggested by IUPAC nomenclature recommendations as true to stoichiometry and structurally neutral. An alternative is name that also conforms to the same criteria is Boron trihydride. However, I would dissuade from using this name to preserve the existing naming style of similar articles, which is employing oxidation states.
Articles that would be affected by this motion are the following:
- Beryllium hydride → Beryllium(II) hydride or Beryllium dihydride
- Covert to disambiguation page directing to Beryllium(II) hydride, and Beryllium monohydride
- Diborane → Boron(III) hydride or Boron trihydride
- Covert Boron hydride to disambiguation page directing to Boranes, Boron(III) hydride, Borane and category:Boranes
- Magnesium hydride → Magnesium(II) hydride or Magnesium dihydride
- Aluminium hydride → Aluminium(III) hydride or Aluminium trihydride
- Calcium hydride → Calcium(II) hydride or Calcium dihydride
- Zinc hydride → Zinc(II) hydride or Zinc dihydride
- Digallane → Gallium(III) hydride or Gallium trihydride
- Covert Gallium hydride to disambiguation page directing to Gallium(III) hydride, and Gallane
- Zinc hydride → Zinc(II) hydride or Zinc dihydride
- Cadmium hydride → Cadmium(II) hydride or Cadmium dihydride
- Thallium hydride → Thallium(III) hydride or Thallium trihydride
Plasmic Physics (talk) 10:11, 2 August 2014 (UTC)
I strongly disagree about the two compounds I do know something about - Diborane and Digallane. These names are the most common and link to the actual structure, whereas Boron(III) hydride and Gallium(III) hydride suggest that the structures are BH3 and GaH3. I suggest you leave well alone. --Bduke (Discussion) 11:21, 2 August 2014 (UTC)
- The issue is whether most readers will know to search for Diborane or Digallane when looking for boron's and gallium's standard saturated hydrides. I can assure you that by established definition, 'Boron(III) hydride' and 'Gallium(III) hydride' make no such suggestions concerning structure. Plasmic Physics (talk) 12:28, 2 August 2014 (UTC)
- A compromise could be the following:
- Beryllium hydride → Beryllium(II) hydride or Beryllium dihydride
- Covert to disambiguation page directing to Beryllium(II) hydride, and Beryllium monohydride
- Create Boron(III) hydride redirecting to Diborane
- Covert Boron hydride to disambiguation page directing to Boranes, Diborane, Borane and category:Boranes
- Magnesium hydride → Magnesium(II) hydride or Magnesium dihydride
- Aluminium hydride → Aluminium(III) hydride or Aluminium trihydride
- Calcium hydride → Calcium(II) hydride or Calcium dihydride
- Zinc hydride → Zinc(II) hydride or Zinc dihydride
- Create Gallium(III) hydride and Gallium trihydride redirecting to Digallane
- Covert Gallium hydride to disambiguation page directing to Digallane, and Gallane
- Zinc hydride → Zinc(II) hydride or Zinc dihydride
- Cadmium hydride → Cadmium(II) hydride or Cadmium dihydride
- Thallium hydride → Thallium(III) hydride or Thallium trihydride
- Beryllium hydride → Beryllium(II) hydride or Beryllium dihydride
- Plasmic Physics (talk) 12:47, 2 August 2014 (UTC)
- In general article names which imply stoichiometry only become important when a number of stoichiometries are possible (e.g. Iron(II) chloride and Iron(III) chloride), I'm not sure this applies for most of these. I don't suggest this out of a mischievous disregard for IUPAC, the reason is practical: Wikipeadia searches primarily work by finding page names and people are lazy - they'll enter the simplest search terms there are. Using common and non-technical names for pages increases the chances of people finding what they want. The pages themselves are where things like stoichiometry should be made clear. Seen through that lens I agree that Boron hydride should be converted to a disambiguation page directing to Boranes, Diborane, Borane and category:Boranes. Likewise Beryllium hydride should become a disambiguation page directing to Beryllium monohydride (as Beryllium(I) hydride) and Beryllium(II) hydride. Project Osprey (talk) 16:05, 2 August 2014 (UTC)
- I do disagree as well. Again, we do not aim to have the article at the (IUPAC-)correct name, we aim at having the article at the common name as 'the public' would know it. This is similar to the discussions earlier (and I thank you for bringing them up here). Many of these compounds are simply known by more common names, or where the IUPAC name is overly precise (beyond what our goal is here). Especially diborane is one of those cases (but the others as well). E.g. Zinc chloride, diethylzinc .. see all the compounds in Category:Zinc_compounds .. the only one that is at 'Zinc(II) XXX' is actually the one you propose to move here, Zinc(II) hydride, but which you personally moved back in January 2013 (and I would suggest that it gets moved back to Zinc hydride). I would look in a chemical catalogue for 'Zinc hydride', I would ask collegues for 'Zinc hydride', I would not even consider to ask for 'zinc two hydride' (and that goes for most of these, the common oxidation state for all of these is the one used for the main article, if 'zinc(I) hydride' is notable and exists (probably this is a fictional example), then the article Zinc(I) hydride can be created, and then still there is no issue with having two names which do not fully line up with each other, the common Zinc hydride (with the common, +2 oxidation state) and the uncommon Zinc(I) hydride (with the uncommon +1 oxidation state and hence the oxidation state notification).
- Please, Plasmic Physics, do not aim to follow IUPAC like this, please take that out of your mind. It is against our manual of style. I am sorry, but there is no consensus for the strict following of IUPAC in naming (and that reasoning goes up our Manual of Style: "A title should be recognizable (as a name for or description of the topic), natural, sufficiently precise, concise, and consistent with the titles of related articles", 'sufficiently precise', not 'precise'), and for many cases it results in original research (not for these simple binary compounds probably, but when the compounds get more complicated, it needs an interpretation of the IUPAC rules beyond 'simple mathematics', see the alkene discussion with user User:Andrewa of some time ago - you really need to know what one reads and how to use them). I suggest, as per Smokefoot below, that you (and probably most editors) stay away from these precise nomenclature and naming issues and just use common names, or leave them as is. --Dirk Beetstra T C 06:27, 3 August 2014 (UTC)
- I honestly feel like I'm repeating myself, this has nothing to do with following IUPAC nomenclature. Can everyone please stop changing the subject. Plasmic Physics (talk) 06:52, 3 August 2014 (UTC)
- So, now explain me why you chose the name 'Boron(III) hydride'? Where did you get that name, and why do you think the article should be moved there? Do you think that the use of 'Boron(III) hydride' is more common than the use of the term '(di-)borane'. Actually, that one is funny, since below you suggest to move Mercury(II) hydride to mercurane(2) ('.. for the sake of conformity, ..'). And by the way, you started of with 'This name is suggested by IUPAC nomenclature recommendations as true to stoichiometry and structurally neutral' - that is not a change of subject if I then say, again, that we do not follow IUPAC like that, we have our own Manual of Style which describes different naming rules, and that was exactly why I said that. Moreover, it results in original research problems. Please, stop precise nomenclature and naming issue discussions. --Dirk Beetstra T C 08:07, 3 August 2014 (UTC)
- I coincidently used the IUPAC recommendations to construct the name 'Boron(III) hydride', for lack of knowledge of an alternative nomenclature system that can be used to construct ordered names. Do you know of an alternative nomenclature system? If so, I'd be happy to investigate it. I already addressed your question in the lead, regarding why I think it should be moved. It has nothing to do with the commonality of a name, it is for practical reasons. If a reader does not know that diborane is the standard structure with the empirical formula BH
3, then the suggested move will automatically redirect them to the correct article. I can't help but notice that you didn't take into account my suggested compromise. I direct you to the five guidelines of WP:Article titles. Note the fifth guideline regarding consistency. They are guidelines, and so it is perfectly acceptable for one to override the ideal of commonality, if pursuing the ideal becomes a liability rather than an asset to the reader with an amateur foreknowledge. Almost every guideline can be used to justify the proposal. Although, I suppose that the argument is how strongly. Plasmic Physics (talk) 11:30, 3 August 2014 (UTC)- Well none of the above should be moved as explained by the others. The policy takes precedence over the guidelines, and that is the use of the common name. You can create redirects for all those suggested names, but please do not move them. Some day I will write an article on MgH gas, it is almost always referred to as MgH in the literature, but we can give it a longer more official name as long as it is actually the common name for the substance. However what is called magnesium hydride is always MgH2. Magnesium(II) hydride is rarely used as a term. Graeme Bartlett (talk) 11:50, 3 August 2014 (UTC)
- I coincidently used the IUPAC recommendations to construct the name 'Boron(III) hydride', for lack of knowledge of an alternative nomenclature system that can be used to construct ordered names. Do you know of an alternative nomenclature system? If so, I'd be happy to investigate it. I already addressed your question in the lead, regarding why I think it should be moved. It has nothing to do with the commonality of a name, it is for practical reasons. If a reader does not know that diborane is the standard structure with the empirical formula BH
- So, now explain me why you chose the name 'Boron(III) hydride'? Where did you get that name, and why do you think the article should be moved there? Do you think that the use of 'Boron(III) hydride' is more common than the use of the term '(di-)borane'. Actually, that one is funny, since below you suggest to move Mercury(II) hydride to mercurane(2) ('.. for the sake of conformity, ..'). And by the way, you started of with 'This name is suggested by IUPAC nomenclature recommendations as true to stoichiometry and structurally neutral' - that is not a change of subject if I then say, again, that we do not follow IUPAC like that, we have our own Manual of Style which describes different naming rules, and that was exactly why I said that. Moreover, it results in original research problems. Please, stop precise nomenclature and naming issue discussions. --Dirk Beetstra T C 08:07, 3 August 2014 (UTC)
- I honestly feel like I'm repeating myself, this has nothing to do with following IUPAC nomenclature. Can everyone please stop changing the subject. Plasmic Physics (talk) 06:52, 3 August 2014 (UTC)
- For clarity, where does it say that policy trumps guidelines? Using your own example, there is revealed a flaw - "Avoid ambiguous abbreviations" - WP:AT. MGH already exists, and MgH is also known as 'magnesium hydride'. Plasmic Physics (talk) 12:05, 3 August 2014 (UTC)
- P.S. Regarding the overriding of commonality: "If it exists, choose an alternative name that the subject is also commonly called in English, albeit not as commonly as the preferred-but-ambiguous title." - WP:AT. Plasmic Physics (talk) 12:40, 3 August 2014 (UTC)
- Take a look at Wikipedia:Policies and guidelines. Just because something is ambiguous does not mean that we have to avoid using the name, especially when one use is far more common than other uses. It is when the different uses are roughly as likely that we need to chose names that will distinguish them. Otherwise the common use get the name, and a hat note will help you find the other use or uses, possibly via a disambiguation page. In some cases it makes sense to have an article on the family of compounds, which would be possible for hydrides of an element. I still support the existence of redirects for official names to the common name. Also there should be redirects for formulas. I am not sure why MgH redirects to MGH. But there is no article or writings on magnesium monohydride yet, so readers have no reason to complain. Graeme Bartlett (talk) 23:58, 3 August 2014 (UTC)
- Where in WP:AT does it draw the distinction? Plasmic Physics (talk) 00:17, 4 August 2014 (UTC)
- Just around what you quoted above. Primary topics and such. --Dirk Beetstra T C 05:51, 4 August 2014 (UTC) I am moving the Thallium hydride part to a new subsection below, needs a bit of different discussion
- Where in WP:AT does it draw the distinction? Plasmic Physics (talk) 00:17, 4 August 2014 (UTC)
- Can we at least agree on the compromised proposal for boron, and gallium, if not for the remainder; and a hat note for beryllium? Plasmic Physics (talk) 21:29, 4 August 2014 (UTC)
- The redirects for boron and gallium are cheap (and I would not really oppose that all the redlinks here are converted to redirects) .. the hatnotes I am really not sure, per WP:UNDUE. --Dirk Beetstra T C 03:28, 5 August 2014 (UTC)
- Can we at least agree on the compromised proposal for boron, and gallium, if not for the remainder; and a hat note for beryllium? Plasmic Physics (talk) 21:29, 4 August 2014 (UTC)
Miscellaneous moves
While not necessary to address the aforementioned issues, for the sake of conformity, Beryllium monohydride and Indium trihydride could also be moved to Beryllium(I) hydride and Indium(III) hydride, respectively. Furthermore, Mercury(II) hydride does not require a structurally neutral name, also for the sake of conformity, I thus suggest moving it Mercurane(2). Plasmic Physics (talk) 10:11, 2 August 2014 (UTC)
- Plasmic, first of all, thanks for asking about some of your thoughts on renaming. My advice is that you avoid issues of nomenclature. Recent initiatives on nomenclature has kicked up a lot controversy and has sucked up too much effort on the part of editors, you included, who could be contributing high level content in other areas. --Smokefoot (talk) 13:46, 2 August 2014 (UTC)
- There is a subtle difference here. While a related topic, the issue regards article naming, and is of a practical nature. It is not about something as relatively trivial as simple nomenclature within the article space. Although, I suppose that it will have to be updated after the moves to reflect the new article names. Plasmic Physics (talk) 14:16, 2 August 2014 (UTC)
- See above. For Mercury(II) hydride, since Hg(I) and Hg(II) are both quite common (both Mercury(II) hydride and Mercury(I) hydride exist), here the indication is necessary. I oppose strongly against Mercurane(2) as the name for the article, again, that is far, far from the common name. --Dirk Beetstra T C 06:27, 3 August 2014 (UTC)
- There is a subtle difference here. While a related topic, the issue regards article naming, and is of a practical nature. It is not about something as relatively trivial as simple nomenclature within the article space. Although, I suppose that it will have to be updated after the moves to reflect the new article names. Plasmic Physics (talk) 14:16, 2 August 2014 (UTC)
- Really? Can you cite that it is most common? Plasmic Physics (talk) 06:52, 3 August 2014 (UTC)
- It strikes me as highly unusual that such an esoteric compound, which has only been known for barely a decade, would already have a most common name. Plasmic Physics (talk) 07:37, 3 August 2014 (UTC)
- Well, Mercurane(2) is the .. IUPAC suggested name, I would however look for .. mercury hydride (and then figure out there is mercury(I) hydride and mercury(II) hydride .. ). Just like I would look for sodium hydride, beryllium hydride, aluminium hydride, phosphane and borane .. As per my suggestion above, if the article was created as Mercury(II) hydride, then the editor doing that found that the .. more common name. Please leave the renaming, naming issues etc. alone. --Dirk Beetstra T C 07:56, 3 August 2014 (UTC)
- Not necessarily - the creator may have simply synthesised the name, and lacked any knowledge of the common name, which seems the more likely explanation when taking into account the circumstances of the article's origin. Plasmic Physics (talk) 10:58, 3 August 2014 (UTC)
- Let's ask User:Whoop whoop pull up to comment on that, and whether they lack that knowledge. All I see that you went into a near edit-war with the editor about a section in this article where you added unreferenced material which they removed again, and later, when you added references, added references which were found to be dubious. Later that whole section was again wholesale removed due to the lack of references capable of supporting the material that you added. I don't know how likely that lack of knowledge on their side is. --Dirk Beetstra T C 11:30, 3 August 2014 (UTC)
- Not necessarily - the creator may have simply synthesised the name, and lacked any knowledge of the common name, which seems the more likely explanation when taking into account the circumstances of the article's origin. Plasmic Physics (talk) 10:58, 3 August 2014 (UTC)
- [1]. From what I remember, the article was created in haste, and out of spite over a content dispute. Plasmic Physics (talk) 12:49, 3 August 2014 (UTC)
- Whatever, you asked for opinions on the namechange, you've got mine and maybe others will comment as well - that article name is just fine. My suggestion however is still, leave naming discussions alone, Plasmic Physics. --Dirk Beetstra T C 03:49, 4 August 2014 (UTC)
- [1]. From what I remember, the article was created in haste, and out of spite over a content dispute. Plasmic Physics (talk) 12:49, 3 August 2014 (UTC)
- And if there is no 'more common' use of either of them, then there is no reason to move either, we leave it at the place where the original author, who thought that that was more recognisable, natural, sufficiently precise, consise and consistent with the titles of related articles, or even where they chose it to be. The request for citations of 'which is more common' is hence circular. --Dirk Beetstra T C 08:07, 3 August 2014 (UTC)
- That reasoning is acceptable, since the suggestion was based on the idea of conformity to a common style, as stated in the lead. Plasmic Physics (talk) 10:58, 3 August 2014 (UTC)
- Although our MOS suggests that conformity, it is by no means a driving force for the choice of article names - the 'conformity' at the moment is that we follow the 'common name', not one type of style systematically (and we don't have to, neither is it going to make any sense in the end as while for Mercury(II) hydride it makes sense to use this name, for Calcium hydride another naming convention makes more sense, and for diborane yet another makes more sense - making it conform to one makes sense on the small scale, but becomes useless if one extrapolates to the other extremes: Carbon(IV) hydride, Oxygen(II) hydride? Use common sense, not rules, we're never getting things to an all-over conform system as that would result in, albeit perfectly correct, impossible and completely uncommon names for articles). --Dirk Beetstra T C 11:30, 3 August 2014 (UTC)
- That reasoning is acceptable, since the suggestion was based on the idea of conformity to a common style, as stated in the lead. Plasmic Physics (talk) 10:58, 3 August 2014 (UTC)
- The guidelines given above, regarding consistency specifies "similar articles", those extremes are exactly that, extremes. They certainly not similar to the same degree. Plasmic Physics (talk) 11:37, 3 August 2014 (UTC)
- Besides, they would be called Hydrogen carbide(-IV) and Hydrogen oxide(-II), since hydrogen is the more electropositive. Plasmic Physics (talk) 11:40, 3 August 2014 (UTC)
- The situation for Calcium hydride (vs. Calcium(II) hydride) is not that dissimilar from Water (vs. Hydrogen oxide(-II), or even ), in both cases the former is, by far, the more commonly used name. That's what our MOS suggests, that is where the article is. Although Calcium(I) hydride may exist, I would even oppose to have it named as a 'you may be looking for ..' above the lede of the article - Calcium hydride (Calcium(II) hydride) is a bulk chemical, all the other hydrides are 'rare' (and some may even lack notability on a Wikipedia level, even if they reasonably exist). --Dirk Beetstra T C 03:49, 4 August 2014 (UTC)
Thallium hydride
What about the case of Thallium hydride? The monohydride is the more stable hydride, and better known than the trihydride, yet the trihydride receives preferential treatment. Plasmic Physics (talk) 00:17, 4 August 2014 (UTC)
- No, Plasmic Physics. That Thallium hydride receives 'preferential treatment' is because you rewrote the article from being about both hydrides to being about the trihydride only. Thallium hydride either probably be about the monohydride (as you suggest the more known hydride, and to my recollection, Th(I) is, reasonably by far, the more common oxidation state), and Thallium(III) hydride should be about the trihydride (but if I am wrong in that, and the Category suggests that, maybe the couple should be 'Thallium(I) hydride' and 'Thallium(III) hydride').
- It is, however, not 'preferential treatment', it is about how the editing process works. Sometimes articles end up in 'really wrong' :places (you may be right about that for Thallium hydride), and then renaming, moving, splitting is an option. However, for the far majority of articles that you name in this section the name is not 'really wrong' - where for Thallium hydride my guess would indeed be 'ThH' (not ThH3), for Magnesium hydride, Calcium hydride, Aluminum hydride, Zinc hydride, and Beryllium hydride that ambiguity does not really exist, those elements have one really major preferred oxidation state that any chemist would expect it to be in (and even high school students - no teacher would teach their students to add 'sodium one sulfate' or 'calcium two carbonate', it would be 'sodium sulfate' or 'calcium carbonate'). --Dirk Beetstra T C 03:49, 4 August 2014 (UTC)
- Thinking about this, as both 'Thallium(I) hydride' as well as 'Thallium(III) hydride' (could) exist (as well as maybe other binary compounds of H and Th), but none of them seem to have independent notability (they seem to be just of academic interest at the moment), maybe the article should be about the (possible) thallium hydrides in general (I do think we should have articles on all 'bulk-element' hydrides), not specifically about one of them (as it seemed to be before the changeover by Plasmic Physics). If one of them gets individual notability that can be handled by a split at that time. --Dirk Beetstra T C 05:51, 4 August 2014 (UTC)
- If something doesn't have "independent notability", why does it have an article? We deal with lots of essoteric and niche topics in chemistry, but WP:GNG still is an overall site guideline. DMacks (talk) 12:52, 4 August 2014 (UTC)
- Agree 100%, especially if the development of these articles is supported by flimsy sources (3rd rate journals and such), On the other hand, one salutary aspect is that this strangeness focuses on unimportant topics, vs threatening important ones. --Smokefoot (talk) 13:38, 4 August 2014 (UTC)
- That is why I say that the article should turn back to be about all 'possible' hydrides of Thallium. The fact that 'they do not exist - or are at least of minor importance' is in itself notable where (practically) all other element-hydrides do exist. The article should never have specialised to a specific hydride. --Dirk Beetstra T C 03:20, 5 August 2014 (UTC)
- Agree that this activity by Plasmic is ill-advised and the results are almost invariably mediocre and suffer from WP:UNDUE. Unfortunate. --Smokefoot (talk) 13:04, 5 August 2014 (UTC)
- If something doesn't have "independent notability", why does it have an article? We deal with lots of essoteric and niche topics in chemistry, but WP:GNG still is an overall site guideline. DMacks (talk) 12:52, 4 August 2014 (UTC)
Mercury hydride is at AfD, having been nominated by user Plasmic Physics. See Wikipedia:Articles for deletion/Mercury hydride. -- 101.117.57.173 (talk) 00:09, 11 August 2014 (UTC)
- I see that, after the above discussion and a discussion with User:John on their talkpage, User:Plasmic Physics unilaterally decided, following the thoughts of the discussion and ignoring the suggestion to leave naming issues alone, that Mercury(I) hydride is not as notable as Mercury(II) hydride, and that therefore Mercury hydride has to be a redirect to the, in their opinion, more common Mercury(II) hydride. When that did not stick, they decided to AfD the article. I am sorry, Plasmic Physics, but I can't decide here whether these actions are WP:POINTy, a failure to drop the WP:STICK, or WP:DISRUPTive. Can you please withdraw the AfD and from now on really leave naming and nomenclature issues alone, at least for a year or two? --Dirk Beetstra T C 03:30, 11 August 2014 (UTC)
- I came into this not knowing any of the history, but looking at the arguments in this specific case, I'm not convinced that Mercury (I) hydride is not a good target for redirect from Mercury hydride, assuming that Plasmic Physics' claims about IUPAC nomenclature don't hold water (and the consensus seems to be that they don't). Mercury hydride is the systematic name for Mercury (I) hydride, according the the article, and according to the IP editor at the AfD, mercury hydride most commonly refers to Mercury (I) hydride in the literature. At the moment, I'm not sure what the disambiguation page is even adding here - most people would need to choose one of the pages at random anyway to try and figure out which hydride they are looking for anyway, since the disambiguation page only disambiguates based on the chemical formula - which is probably not going to help non-chemists disambiguate between the two, and chemists would likely find redundant anyway since the article titles are disambiguated by oxidation state anyway. 0x0077BE [talk/contrib] 03:43, 11 August 2014 (UTC)
- The disambiguation page is there to show that there are two pages that 'Mercury hydride' could refer to. I don't think either has a significant significance over the other, so solutions could be to either merge the two into Mercury hydride (which may become somewhat confusing if there is enough to tell about either), or have both separated with a disambig in front of it. I do not believe there is a primary topic here at this time, I might only agree that the disambiguation could do a better job in disambiguation. I just think that this is an 'extrapolation' by User:Plasmic Physics from the naming and organisation other hydrides, where the situations are obviously different, and that that then also has to be implemented here. --Dirk Beetstra T C 06:14, 11 August 2014 (UTC)
- I came into this not knowing any of the history, but looking at the arguments in this specific case, I'm not convinced that Mercury (I) hydride is not a good target for redirect from Mercury hydride, assuming that Plasmic Physics' claims about IUPAC nomenclature don't hold water (and the consensus seems to be that they don't). Mercury hydride is the systematic name for Mercury (I) hydride, according the the article, and according to the IP editor at the AfD, mercury hydride most commonly refers to Mercury (I) hydride in the literature. At the moment, I'm not sure what the disambiguation page is even adding here - most people would need to choose one of the pages at random anyway to try and figure out which hydride they are looking for anyway, since the disambiguation page only disambiguates based on the chemical formula - which is probably not going to help non-chemists disambiguate between the two, and chemists would likely find redundant anyway since the article titles are disambiguated by oxidation state anyway. 0x0077BE [talk/contrib] 03:43, 11 August 2014 (UTC)
Chlorine Peroxide
Chlorine peroxide should be of this form: Cl-O-O-Cl but instead it redirects to O=Cl=O or chlorine dioxide. A peroxide is by definition of this form: A-O-O-A or A-O-O-B where A and B are any atom besides oxygen or flourine(F-O-O-F is Dioxygen Diflouride with each O in a +1 oxidation state). The O-O single bond is what differentiates peroxides from other oxides. Chlorine peroxide should redirect to Dichlorine Dioxide instead of Chlorine Dioxide and the infobox for Dichlorine Dioxide should be edited in the Other Names section to include Chlorine Peroxide since it is in fact a peroxide.Caters1 (talk) 01:23, 12 August 2014 (UTC)
- The spirit of the redirect is to help readers vs satisfy the fastidious tendencies of chemists. Readers might think that ClO2 is a cyclic peroxide. Wikipedia would welcome an article on ClOOCl (RN 12292-23-8). Key to creating an article are WP:SECONDARY references to establish notability, and here are two reviews:
- "Oxygen compounds of halogens X2O2(X is a halogen atom" doi 10.1070/RC2002v071n02ABEH000684
- "UV Photolysis of ClOOCl and the Ozone Hole" DOI: 10.1002/asia.201100151
Happy editing.--Smokefoot (talk) 02:52, 12 August 2014 (UTC)
- ClO2 indeed has a resonance structure in which there is a peroxide bond in the ring. However this is very unstable, more so than O=Cl=O. This is why CO2 does not exist in a cyclic form is because cyclic peroxides are unstable in normal conditions, especially ones in 3 membered rings.
- Because of this, acyclic peroxides are preferred over cyclic peroxides and so chlorine peroxide would most likely be Cl-O-O-Cl. It is the free radical form of this, that is 2 Cl-O + CO2 that causes there to be the ozone hole(and not the peroxide itself) because Cl-O(chlorine monoxide) violently reacts with Ozone to form Chlorine Dioxide and O2 and CO2 is easily(more easily than O2) photolysed into CO and O(atomic oxygen) both of which violently react with the Ozone. CO reacts with O3 to form CO2 and O2(similar reaction to the one with chlorine monoxide) and atomic oxygen reacts with the Ozone to form 2 O2 molecules.
- Thus because of acyclic peroxides being the prefered type of peroxide the Chlorine Peroxide page redirect is redirecting to the wrong page.Caters1 (talk) 03:07, 12 August 2014 (UTC)
- Dear Caters1: recheck meaning of resonance structure. --Smokefoot (talk) 10:57, 12 August 2014 (UTC)
- We do have an article on ClOOCl at dichlorine dioxide. I can't see anyone objecting to you changing the redirect. Project Osprey (talk) 09:18, 12 August 2014 (UTC)
- I have made an improved page at ClO dimer using one of the suggested references that actually includes info that I can find online. I have changed redirects all round. Graeme Bartlett (talk) 10:31, 12 August 2014 (UTC)
- Resonance structure means "If you move these electrons onto here and form a new bond or just move the electrons(like in forming an alkoxide from a ketone or aldehyde for instance) you get a different structure of the same molecule" Thus ClO2 has a cyclic resonance structure because you can move the pi electrons onto the oxygen atoms and form a peroxide bond to get a cyclic peroxide. It also has acyclic resonance structures much like those of CO2.Caters1 (talk) 14:16, 12 August 2014 (UTC)
pertechnetic acid
i think an image for technetic(V) acid got lost 24.131.80.54 (talk) 03:40, 9 August 2014 (UTC)
- Do you mean Pertechnetic acid? The revision history doesn't seem to indicate that any images were lost. Care to enlighten? 0x0077BE [talk/contrib] 03:07, 11 August 2014 (UTC)
- Something is wrong with the image used in the article. File:Pertechnetic_acid.png is used in the infobox but it does not display properly for me either. ChemNerd (talk) 11:22, 11 August 2014 (UTC)
- Fixed. Project Osprey (talk) 12:06, 11 August 2014 (UTC)
- In the new image, File:Pertechnetic acid 3D ball.png, shouldn't three of the Tc-O bonds be double bonds, rather than only two of them? And in the old image, File:Pertechnetic_acid.png (which for some reason is displaying properly for me now when I look at the article history) is there an oxygen atom missing, or is it just hidden behind the Tc atom? ChemNerd (talk) 17:10, 11 August 2014 (UTC)
- Actually, in the new image there are 3 double bonds; it's just hard to see because of how its orientated. No idea what was happening in the original image. I'm not actually the one making these btw, I just find them on wikimedia. Project Osprey (talk) 21:28, 11 August 2014 (UTC)
- Thanks. I see now what I missed in Pertechnetic acid 3D ball.png ChemNerd (talk) 20:21, 12 August 2014 (UTC)
- Fixed. Project Osprey (talk) 12:06, 11 August 2014 (UTC)
- Something is wrong with the image used in the article. File:Pertechnetic_acid.png is used in the infobox but it does not display properly for me either. ChemNerd (talk) 11:22, 11 August 2014 (UTC)
Base
I am looking for more opinions on whether to call substances that can react with the naked proton a base or not. In Talk:Methylidyne radical User:Plasmic Physics considers that Methylidyne radical and even argon and helium are bases because they can react with a proton (in the gas phase). However I think we should only state that the substance is a base if other reliable sources say so. What do others think? Should every hydride that can part with a proton or accept a proton be called amphoteric with an unsourced Amphotericity section? Graeme Bartlett (talk) 11:04, 13 August 2014 (UTC)
- "Naked proton" is hyper specific. Argon and helium being bases are extreme examples, which you raised to ridicule the implications of the argument. Yes, technically helium is the conjugate base of the helium hydride ion, but it is such a weak base, that its basicity is not notable. Methylidyne is not in the same ball-park; it is not a fair comparison.
- The basicity of methylidyne is a logical implication of the conjugate acid-base model of Brønsted–Lowry acid–base theory, and as such, it is covered by "uncontroversial knowledge" under WP:SCICITE. The Amphotericity section describes methylidyne's ability to act as both a Lewis acid, and a Lewis base. As you all know, protonation within the Brønsted–Lowry acid–base theory is a particular type of adduction, where the hypothetical proton acts as the Lewis acid. The 'adduct' of methylidyne and a proton is known to exist. Furthermore, the section is making no speculative commentary which cannot be independently confirmed to comply with the BL theory. Another important concept is proton affinity, which specifically describes the helium hydride ion as an extreme example. Now the section does not say that methylidyne is more basic than it is acidic, only that both types of character are exhibited. Plasmic Physics (talk) 11:57, 13 August 2014 (UTC)
- H+ can accept H+ in proton–proton chain reactions to give D+. Can I therefore argue that acid has Lewis basic character with a pKa of infinity? Project Osprey (talk) 12:17, 13 August 2014 (UTC)
- No, that is non-sense. You example is concerns nuclear physics, not a chemical reaction. It's like counting whales among the number of fish in the ocean. Just because both swim... Plasmic Physics (talk) 12:21, 13 August 2014 (UTC)
- No, it is actually pretty funny. Please turn on your humor detector ;-) Boghog (talk) 13:59, 13 August 2014 (UTC)
- What am I missing, because I don't get it? Plasmic Physics (talk) 14:21, 13 August 2014 (UTC)
- Unless I am terribly mistaken, the above is a subtle example of surreal humour. Boghog (talk) 14:44, 13 August 2014 (UTC)
- That explains it - having Asperger's syndrome means that I'm naturally immune to surreal humour. Plasmic Physics (talk) 22:48, 13 August 2014 (UTC)
- Would Plasmic agree to discuss proposed edits on Talk pages? If consensus is achieved, the changes could be implemented by another editor. End of problem. Otherwise we get caught up in these litigious and time-consuming discussions. Plasmic's contributions to chemistry are often of highly debatable quality (see discussion above), being supported by his own interpretations (synthesis) or flimsy referencing. Through vetting on a talk page and then seeking consensus, Plasmic could still contribute and the real mission here = serving readers - could move forward. --Smokefoot (talk) 13:50, 13 August 2014 (UTC)
- I agreed to discussion of controversial edits; amending my own contributions is hardly controversial. And, all these "litigious and time-consuming discussions" are in actuality the result of consensus building. Plasmic Physics (talk) 14:17, 13 August 2014 (UTC)
- I would argue we already have a consensus. You've broached the concept of the amphotericity of radicals before in Methylidenecarbene [[2]] and Methylene [[3]]. I seem to remember that in both cases the concept was discussed here and that the consensus was that it was inappropriate (so Smokefoot is right to desire a discussion on talk pages, it would make such records easier to find). Amphoteric is a poor description, radicals don't have special reactivity with both acids and bases, they have wild and all-inclusive reactivity towards almost everything, including each other. That's the sort of chemistry we should be describing. Project Osprey (talk) 14:40, 13 August 2014 (UTC)
- I'm not certain about what you're saying. Are you making the sweeping statement that for all radicals, that their radical chemistry is solely notable in such a way that all other chemistry should be ignored? What then of the superoxide ion, and many others? Should we proceed to remove any content for them which does not pertain to their radicality? Plasmic Physics (talk) 22:44, 13 August 2014 (UTC)
- We did start to discuss the issue on the talk page. But few others participate there, and more people join in here on the project page. I brought in the example of argon to say we would not call it a base, but Plasmic then brought up helium. Any way that is a side issue to whether Brønsted–Lowry acid–base theory can be used to draw a previously unpublished conclusion to include in an article. The idea here is to be constructive rather than litigious. Since there are more around here that actually know Chemistry. Graeme Bartlett (talk) 12:09, 14 August 2014 (UTC)
- Indeed. As I see it, it is a fair conclusion, comparable to the statement "The Oxford Scientific Dictionary will fall to the ground with an acceleration of 9.8 m s-2 , if it is dropped from a height", derived from Newton's theory of gravity. Now, I can almost guarantee, that statement will not be published anywhere. Plasmic Physics (talk) 21:42, 14 August 2014 (UTC)
- However we do not include this fact on the Oxford Scientific Dictionary article on Wikipedia. WP:OR allows some application of simple logic, such as counting, adding, converting units, reading a map, but it does not include chemistry related derivations. I would say even working out equilibrium ratios at different temperatures would be WP:OR. Graeme Bartlett (talk) 22:27, 14 August 2014 (UTC)
- Indeed. As I see it, it is a fair conclusion, comparable to the statement "The Oxford Scientific Dictionary will fall to the ground with an acceleration of 9.8 m s-2 , if it is dropped from a height", derived from Newton's theory of gravity. Now, I can almost guarantee, that statement will not be published anywhere. Plasmic Physics (talk) 21:42, 14 August 2014 (UTC)
- Yes, but WP:SCICITE overrides WP:OR. Plasmic Physics (talk) 23:04, 14 August 2014 (UTC)
- Wrong. First of all WP:OR is a policy while WP:SCICITE is a guideline. Second, WP:SCICITE does not contradict WP:OR. All WP:SCICITE says is that is not necessary to document each and every uncontroversial statement as long as a general source is included that can be used to support these statements. Further WP:SCICITE states that
material whose verifiability has been challenged, should always be accompanied by an inline citation
. The verifiability of your assertion that Methylidyne radical can be classified as a base has been challenged. If you want to include a statement to that effect, you now must provide a reliable source to support that statement. Boghog (talk) 05:51, 15 August 2014 (UTC)- It should be all too easy to provide an inline citation to BL theory. Plasmic Physics (talk) 06:42, 15 August 2014 (UTC)
- You completely missed the point. You assertion has been challenged. If you want the statement to be included in the article, you must provide a reliable source that directly supports your statement. Full stop. Boghog (talk) 06:55, 15 August 2014 (UTC)
- It should be all too easy to provide an inline citation to BL theory. Plasmic Physics (talk) 06:42, 15 August 2014 (UTC)
- Wrong. First of all WP:OR is a policy while WP:SCICITE is a guideline. Second, WP:SCICITE does not contradict WP:OR. All WP:SCICITE says is that is not necessary to document each and every uncontroversial statement as long as a general source is included that can be used to support these statements. Further WP:SCICITE states that
- Obviously we have a contrasting interpretation of WP:SCICITE. Lets just agree to disagree, shall we? Plasmic Physics (talk) 09:53, 15 August 2014 (UTC)
- When it comes to Wikipedia policy, I will not agree to disagree. WP:OR is policy and must be followed. Your interpretation of the WP:SCICITE guideline is WP:WIKILAWYERING and is seriously flawed. Furthermore your actions directly contravene proposed solution that you agreed to above. If other editors question your contribution, the contribution by definition become controversial and you then are obligated to proved reliable sources to support your contribution. So far you have provided no source that directly supports that Methylidyne radical can act as a proton acceptor. Unless you can supply such a source, your edits will be reverted. Boghog (talk) 10:30, 15 August 2014 (UTC)
- In order for Methylidyne radical to act as a proton accepting base, the product CH2+ should be stable. The following source suggests CH2+ is not stable:
- Wetzel TL, Welton RF, Thomas EW, Borkman RF, Moran TF (1993). "The elusive CH2+ ion". Journal of Physics B: Atomic, Molecular and Optical Physics. 26 (1). doi:10.1088/0953-4075/26/1/005.
Experiments with an electron cyclotron resonance ion source have been employed in a hunt for the elusive CH2+ ion. The authors' experiments do not find evidence for the existence of stable CH2+ ions. Different levels of ab initio molecular orbital theory have been employed to obtain potential energy curves for CH2+. Although SCF calculations show a small minimum in the potential, post-Hartree-Fock computations indicate CH2+ states to be repulsive.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Hence it is very doubtful that methylidyne radical could act as a base. Boghog (talk) 06:29, 15 August 2014 (UTC)
- Where did you hear that the conjugate acid needs to be stable? There are plenty of known bases, in which the CA is unstable. Post protonation reactions have no effect on the qualitative basicity of the original reacting species. The tetrahydridoborate anion is a well known superbase, the protonated conjugate acid is highly unstable, ejecting a dihydrogen molecule. This neutralization reaction is key to explaining the hydrolysis of tetrahydridoborate salts. Plasmic Physics (talk) 06:42, 15 August 2014 (UTC)
- Agreed that the conjugate acid can be unstable, but it should at least be a local minimum along the potential energy surface so that has a finite life time. The above source suggests that the CH2+ configuration is repulsive (i.e., not a local minimum). Boghog (talk) 07:02, 15 August 2014 (UTC)
- Where did you hear that the conjugate acid needs to be stable? There are plenty of known bases, in which the CA is unstable. Post protonation reactions have no effect on the qualitative basicity of the original reacting species. The tetrahydridoborate anion is a well known superbase, the protonated conjugate acid is highly unstable, ejecting a dihydrogen molecule. This neutralization reaction is key to explaining the hydrolysis of tetrahydridoborate salts. Plasmic Physics (talk) 06:42, 15 August 2014 (UTC)
- In order for Methylidyne radical to act as a proton accepting base, the product CH2+ should be stable. The following source suggests CH2+ is not stable:
- Here is a more recent reference that shows that it is a minimum: Wang, Haiming; Neese, Christopher Neese; Morong, Christopher P.; Kleshcheva, Maria; Oka, Takeshi (3 October 2013). "High-Resolution Near-Infrared Spectroscopy of CH+
2 and Its Deuterated Isotopologues". The Journal of Physical Chemistry A. 117 (39). ACS Publications: 9908–9918. doi:10.1021/jp3128803.. - Within the article there is also says that a group identified it from a spectrum in 1991 which predates your source. Plasmic Physics (talk) 09:53, 15 August 2014 (UTC)
- The conflicting literature above supports that the existence of CH+
2 is controversial. Furthermore the references that you have provided above only indicate that it is theoretically possible thatCH+CH could act as a base. A reliable source that directly states that
2CH+CH can act as a proton acceptor is required. Otherwise your assertion becomes WP:OR and has absolutely no place in Wikipedia. Boghog (talk) 10:41, 15 August 2014 (UTC)
2
- The conflicting literature above supports that the existence of CH+
- Here is a more recent reference that shows that it is a minimum: Wang, Haiming; Neese, Christopher Neese; Morong, Christopher P.; Kleshcheva, Maria; Oka, Takeshi (3 October 2013). "High-Resolution Near-Infrared Spectroscopy of CH+
- (You mean CH?) I could rewrite it as a juxtaposition of standalone sentences, which are all referenceable in their own right. Leaving the reader to draw a conclusion to the same effect. Maybe something like "Methylidyne has Lewis base character", "A proton has Lewis acid character", "CH+
2 is known to exist", "A proton is both a Lewis acid and a Brønsted acid", etc. - Almost nothing would be achieved by reversion, you would removed a other information which is explicitly sourceable, and you'd still be left with a section which contains information on its Arrhenius acidity, which I amended. If you want to make a successful alteration, I would suggest careful dissection of the offending content rather than cauterising it. However, it's best to leave that decision until we've finished the preceding discussion on whether it can simply be rewritten. Plasmic Physics (talk) 11:58, 15 August 2014 (UTC)
- (You mean CH?) I could rewrite it as a juxtaposition of standalone sentences, which are all referenceable in their own right. Leaving the reader to draw a conclusion to the same effect. Maybe something like "Methylidyne has Lewis base character", "A proton has Lewis acid character", "CH+
- By reverting your contribution, we would be eliminating content which is neither verifiable nor notable and bring the article into conformity with long standing Wikipedia policies. Wang et al., 2013 does support the existence of the methylene cation CH+
2. However we still need a reliable source to support that methylidyne radical (CH) will react with protons to form methylene cation (CH+
2). Boghog (talk)- Just to clarify: the Wetzel paper[4] concerns the doubly charged CH2+
not CH+
2. The latter is stable.[5] --Kkmurray (talk) 21:27, 15 August 2014 (UTC)- My mistake. Thanks Kkmurray or clearing that up. Boghog (talk) 21:36, 15 August 2014 (UTC)
- Just to clarify: the Wetzel paper[4] concerns the doubly charged CH2+
- @Plasmic Physics: Because your contributions to the Methylidyne radical article are controversial and you have not provided reliable sources to support your contributions, I have reverted them. Per the proposed solution that you agreed to above, you must provide a reliable source that directly supports your assertion before re-adding this material. Boghog (talk) 21:32, 15 August 2014 (UTC)
- By reverting your contribution, we would be eliminating content which is neither verifiable nor notable and bring the article into conformity with long standing Wikipedia policies. Wang et al., 2013 does support the existence of the methylene cation CH+
Compeed (hydrocolloid technology)
Please check if the chemistry behind Compeed plaster is properly described in Techology part. I was using cited sources (patents, Compeed description at Expainthatstuff article]) as well as Amazon ingredients data. -- TigerInWoods (talk) 13:15, 17 August 2014 (UTC)
smart rubber needs a rewrite
Cross-posting from WikiProject Polymers to get a wider audience: Section title:"Smart rubber page is hard to read". Most of the content of the article appears to have been copied from a single article, on a single type of polymer. Wasn't general at all, and virtually no context. Also no inline citations, so hard to see where most material was being sourced from. I took a stab at a major rewrite myself, but it could probably use some more work. (+)H3N-Protein\Chemist-CO2(-) 22:47, 21 August 2014 (UTC)
Hydronium is an Oxycation and technically CO is as well
Oxycation means positive charge on oxygen. Oxygen hates this which is why hydronium is an ion in a complex of ions which itself is in a complex of ions and so on and so forth is so that the positive charge is spread instead of localized to 1 oxygen atom.
Hydronium is H3O+ and the positive charge is on the oxygen.
3 bonds + 1 lone pair = +1 charge on oxygen
CO has this as well because of the 2.5 bond and so despite the oxygen being more electronegative it is positive and the C is negative because of this:
3 bonds + 1 lone pair
for carbon this means a -1 charge and for oxygen it means a +1 charge
The fact that it is the oxygen that is positive in both of these cases makes them oxycations. Caters1 (talk) 12:57, 31 July 2014 (UTC)
- This is not the place to speculate. We work on sources. However, you will not find a source that says that CO is an oxycation, because it is not. To be an oxycation it has to be a cation. To be a cation, it has to be an ion and CO is not. Also the O in CO is not that -ve. The dipole moment is quite small. --Bduke (Discussion) 20:54, 31 July 2014 (UTC)
- To be an ion, the species must, by definition have an overall charge. Carbon monoxide lacks this key quality. Plasmic Physics (talk) 21:07, 31 July 2014 (UTC)
- CO has a 2.5 bond which makes there be a positive charge on the oxygen since it is the O's lone pair that is used to form the bond while the C's lone pair stays there. Thus the carbon becomes negative(-1 formal charge, not partially negative) and the oxygen has a +1 formal charge which it hates.
- Thus while it is neutral overall to be an oxycation it does not mean the whole molecule has to have a positive charge. Oxycation literally translates to "Positively Charged Oxygen" which is all it means.Caters1 (talk) 02:00, 1 August 2014 (UTC)
- Carbon monoxide has a fairly established triple bond. But let's step back...what's your point here? Yes, the O of CO has a formal charge of +1. No, nobody here really cares about specific identifying terms for it unless a reliable source says so. No, the oxygen is not actually a +1 ion (bond polarization counteracts it), and the molecule is not nearly as polar as the formal charges would suggest. DMacks (talk) 04:04, 1 August 2014 (UTC)
- Thus while it is neutral overall to be an oxycation it does not mean the whole molecule has to have a positive charge. Oxycation literally translates to "Positively Charged Oxygen" which is all it means.Caters1 (talk) 02:00, 1 August 2014 (UTC)
- Actually, that is incorrect. Even if 'oxycation' is literally translated (despite it already being in english), that is not its definition. An oxycation is any cation which contains oxygen, and does in fact require an overall charge on the species. Plasmic Physics (talk) 06:15, 1 August 2014 (UTC)
- Chemists always talk about it in terms of formal charge and when they say oxycation, they don't necessarily mean positively charged molecule with oxygen. In fact this is an uncommon definition in chemistry, especially organic chemistry. Instead they more often mean instead of a +1 ion with oxygen, a molecule that may or may not be neutral but has a positively charged oxygen.
- Actually, that is incorrect. Even if 'oxycation' is literally translated (despite it already being in english), that is not its definition. An oxycation is any cation which contains oxygen, and does in fact require an overall charge on the species. Plasmic Physics (talk) 06:15, 1 August 2014 (UTC)
- CO has 2 resonance structures that follow the octet rule and 1 that doesn't. They are these [:C=O(with 2 lone pairs on the O) <-> -^:C-=O:^+(which follow the octet rule) and <-> +^:C-O^-(with 3 lone pairs of electrons that doesn't follow the octet rule)]
- The one with a triple bond has a carbanion(negatively charged carbon) and an oxycation(positively charged oxygen). The one with the single bond has a carbocation(positively charged carbon) and an oxyanion(negatively charged oxygen). All of these are based on formal charges and not partial charges caused by polarity. The carbon in CO always has 1 lone pair of electrons. There is no case where the double bonded one is like this (0 lone pairs)C=O(with 2 lone pairs) as this would make carbon have a +2 charge which is even more unstable than a +1 charge for something not so electropositive. Carbon isn't so electronegative either, especially compared to hydrogen where there is like a .04(maybe .4) difference and so it is at best mildly polar those hydrocarbons and also a -2 charge would be even more unstable for carbon than a -1 charge because of this.Caters1 (talk) 19:09, 1 August 2014 (UTC)
- [(:)C]=[O(::)] is not octet stable (it only follows the strict "don't exceed octet" part, not the "meet octet" maximal stability part). It's closer but still non-compliant in the same sense as +[(:)C]–[O(:::)]+ is non-compliant. But once you're just making up things as you go along, you would need to consider all sorts of octet-deficient and multiply-charged cases rather than only the "most likely/reasonable" ones in your analysis. Again again, nobody is disagreeing that CO has a formal O+1 and that this is an atom that is cationic, and nobody seems to be willing to change their opinion of the definition of contracted term "oxycation". Unless you or someone else wishes to raise a new point, I agree that this section should (and probably should have a while ago) be hatted so we don't spend more time not-writing-an-encyclopedia. DMacks (talk) 20:29, 1 August 2014 (UTC)
- I have watched organic chemistry videos and when they say -cation they mean positively charged atom so carbocation = positively charged carbon, not positively charged molecule that contains carbon. They say similar things for carbanion, oxyanion, and oxycation with -anion meaning negatively charged atom, not molecule. Some inorganic chemists say the same thing and so would agree with my statement that CO has in the resonance structure with a triple bond a carbanion and an oxycation. The sites where I have watched these organic chemistry videos that say carbocation = positively charged carbon, carbanion = negatively charged carbon, oxyanion = negatively charged oxygen, and oxycation = positively charged oxygen are Youtube and Khan Academy, with Khan Academy being more reliable.Caters1 (talk) 21:24, 1 August 2014 (UTC)
- https://www.khanacademy.org/science/organic-chemistry/organic-structures/formal-charge-resonance/v/formal-charge-i says that carbocation mean positively charged carbon atom within a molecule and carbanion means negatively charged carbon atom within a molecule.
- https://www.khanacademy.org/science/organic-chemistry/new-topic-2014-06-13T19:24:28.593Z/formation-of-enolate-anions/v/enolate-formation-from-aldehydes and
- https://www.khanacademy.org/science/organic-chemistry/new-topic-2014-06-13T19:24:28.593Z/formation-of-enolate-anions/v/enolate-formation-from-ketones both say that an oxyanion is a negatively charged oxygen atom within a molecule(just like with the carbocation and carbanion the overall charge on the molecule could be positive, negative, or neutral).
- I have watched organic chemistry videos and when they say -cation they mean positively charged atom so carbocation = positively charged carbon, not positively charged molecule that contains carbon. They say similar things for carbanion, oxyanion, and oxycation with -anion meaning negatively charged atom, not molecule. Some inorganic chemists say the same thing and so would agree with my statement that CO has in the resonance structure with a triple bond a carbanion and an oxycation. The sites where I have watched these organic chemistry videos that say carbocation = positively charged carbon, carbanion = negatively charged carbon, oxyanion = negatively charged oxygen, and oxycation = positively charged oxygen are Youtube and Khan Academy, with Khan Academy being more reliable.Caters1 (talk) 21:24, 1 August 2014 (UTC)
- [(:)C]=[O(::)] is not octet stable (it only follows the strict "don't exceed octet" part, not the "meet octet" maximal stability part). It's closer but still non-compliant in the same sense as +[(:)C]–[O(:::)]+ is non-compliant. But once you're just making up things as you go along, you would need to consider all sorts of octet-deficient and multiply-charged cases rather than only the "most likely/reasonable" ones in your analysis. Again again, nobody is disagreeing that CO has a formal O+1 and that this is an atom that is cationic, and nobody seems to be willing to change their opinion of the definition of contracted term "oxycation". Unless you or someone else wishes to raise a new point, I agree that this section should (and probably should have a while ago) be hatted so we don't spend more time not-writing-an-encyclopedia. DMacks (talk) 20:29, 1 August 2014 (UTC)
- The one with a triple bond has a carbanion(negatively charged carbon) and an oxycation(positively charged oxygen). The one with the single bond has a carbocation(positively charged carbon) and an oxyanion(negatively charged oxygen). All of these are based on formal charges and not partial charges caused by polarity. The carbon in CO always has 1 lone pair of electrons. There is no case where the double bonded one is like this (0 lone pairs)C=O(with 2 lone pairs) as this would make carbon have a +2 charge which is even more unstable than a +1 charge for something not so electropositive. Carbon isn't so electronegative either, especially compared to hydrogen where there is like a .04(maybe .4) difference and so it is at best mildly polar those hydrocarbons and also a -2 charge would be even more unstable for carbon than a -1 charge because of this.Caters1 (talk) 19:09, 1 August 2014 (UTC)
- By this logic oxycation should mean positively charged oxygen atom, not positively charged molecule that contains oxygen. Caters1 (talk) 18:29, 11 August 2014 (UTC)
- And both :C=O(::) and :C-=O: are octet stable. Why? The O has an octet in both cases. C can have less than an octet and that doesn't matter. O can't really have less than an octet though even when talking about a peroxide bond which is why an alkoxide has 3 lone pairs of electrons on the O. This is also why the single bond resonance structure of O2 has 2 negatively charged oxygens both with 3 lone pairs of electrons (:::)O-O(:::)
- Your single bond resonance structure of O2 dies not exist, You show 14 valence electrons, not 12. It is O22-. I want to make a general point. The term oxycation or oxyanion can not be used just because one resonance structure is +ve or -ve, unless that structure is dominant. The use of these terms also has to be backed up by experimental information. It is not appropriate to use the terms for CO because the dipole moment of CO is extremely small. It is not a polar molecule. --Bduke (Discussion) 21:10, 25 August 2014 (UTC)
- And both :C=O(::) and :C-=O: are octet stable. Why? The O has an octet in both cases. C can have less than an octet and that doesn't matter. O can't really have less than an octet though even when talking about a peroxide bond which is why an alkoxide has 3 lone pairs of electrons on the O. This is also why the single bond resonance structure of O2 has 2 negatively charged oxygens both with 3 lone pairs of electrons (:::)O-O(:::)
- By this logic oxycation should mean positively charged oxygen atom, not positively charged molecule that contains oxygen. Caters1 (talk) 18:29, 11 August 2014 (UTC)
Sulfonyl and Sulfone
As completely changing the definition a functional group might prove contentious, I thought I’d better bring this up here first.
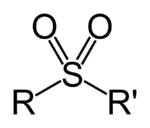
Currently we describe the functional groups Sulfonyl and Sulfone as being the same thing i.e. R-S(=O)2-R'. Both pages use the same image displayed on the right. The IUPAC goldbook has no definition for sulfonyl but does list RS(=O)2R for sulfones and RS(=O)2OH for sulfonic acids. I think sulfonyl should probably refer to sulfonyl halides RS(=O)2X and that it should be merged into that page. I’d be interested in hearing any other opinions though. Project Osprey (talk) 13:05, 29 August 2014 (UTC)
- The former refers to the functional group, the latter refers to the parent compound, in the same way as "carboxyl" and "carboxylic acid" relate. Plasmic Physics (talk) 03:14, 30 August 2014 (UTC)
- If that's true then the page still need to be changed; to define sulfonyl as being RS(=O)2Y, where Y = C, OH, X etc. Project Osprey (talk) 12:53, 30 August 2014 (UTC)
The sulfonyl can also be considered a fragment in inorganic compounds, where it is more commonly termed 'sulfuryl', such as in sulfuryl chloride and sulfuryl fluoride. I quite like the fact Wikipedia has separate articles for related but distinct fragments, functional groups and classes of molecules such as these. It makes it easier for a reader to search for the word when they encounter it, and find an article that quickly and unambiguously defines the word, provides a structure and links to specific examples or related articles. I actually think merging such articles does a serious disservice to our readers. If I don't know what 'sulfonyl' means and I get redirected to sulfone, I will have to read a load of irrelevant information about sulfones before getting the definition I actually want. I feel we should change our policy on this and fix all such situations throughout chemistry articles on Wikipedia. --Ben (talk) 17:18, 30 August 2014 (UTC)
- I'm fine with having pages on fragments, I just don't think this one is described properly. We already have a page on Sulfuryl. Project Osprey (talk) 19:39, 30 August 2014 (UTC)
Acid-base indicators: a graphic view
I have created a graphic view of the pH indicators giving their operation range and the associated colour change. The image can be found here: https://commons.wikimedia.org/wiki/File:A_graphic_view_of_Acid-Base_Indicators.svg
I would like to know where the image can be placed for a better usage. (Damitr (talk) 7:59 am, Today (UTC−4))
- It's an impressive graphic! pH indicator would be one good place to use it. By the way, the way I see it rendered on my Windows/Chrome system, three of the indicator names are displaced to, or beyond, the left edge of the image. As it's SVG, this could easily be corrected. Maproom (talk) 12:29, 2 September 2014 (UTC)
- It looks good. Though thymol blue is missing the base side of its colour change. For Crystal violet I cannot see green or violet in the colour band. There are several indicaters here with no article, for example Methyl green. Graeme Bartlett (talk) 21:22, 18 September 2014 (UTC)
Water ionizer appears to be a page on some form of quackery. It's not a new page but its recently had a huge surge in page views, currently ~15,000 per day, making it the most viewed chemistry page so far this month. I'm going to try and clean it up but the science is shaky and WP:MEDRS will likely raise its head, so if anyone is free I'd appreciate a hand. Project Osprey (talk) 22:11, 18 September 2014 (UTC)
- Looks like fringe science that would not be cured even by Earthing Therapy, although boriding certain editors might be worth considering. More seriously, you could point out that the autoionization constant for water is a constant, see Self-ionization of water.--Smokefoot (talk) 22:27, 18 September 2014 (UTC)
- Oh its plenty wacky, for example autoionization wouldn't apply as this device only produces negative ions. I'm guessing that the 'science' might actually be electrolysis to produce small amounts of hydrogen peroxide which then breaks down to form all sorts, with a net increase in pH. That would make it a hybrid between hydrogen peroxide therapy and electrolysed water. A perfect irony is that its marketed as an anti-oxidant. It has the usual crazy claims, my favourite being that it can protect you from nuclear fallout.
Dear chemistry experts: Apparently these two pages are about the same topic. Is any of the information/sourcing in the draft suitable to expand the stub? —Anne Delong (talk) 02:27, 18 September 2014 (UTC)
- Smokefoot has taken care of this with a merge. Graeme Bartlett (talk) 21:23, 18 September 2014 (UTC)
- Sorry if I acted too swiftly, but the merge seemed pretty obvious. I am still refining the article, parent boriding, but others are welcome to join in. It seems to be an important topic, so we can all thank Anne for rescuing another topic. --Smokefoot (talk) 22:27, 18 September 2014 (UTC)
- I have moved the draft to mainspace and added the appropriate "merge to" and "merge from" templates to preserve attribution. One more off my list... —Anne Delong (talk) 12:09, 20 September 2014 (UTC)
npp for category tool
Please comment. Gryllida (talk) 23:38, 23 September 2014 (UTC)
The usage of Carbon fiber (edit | talk | history | protect | delete | links | watch | logs | views) is under discussion, see talk:carbon (fiber) -- 65.94.171.225 (talk) 04:23, 25 September 2014 (UTC)
template should contain sections on toxicology and environment
Hi, IMHO a general problem with chemistry articles is, that they have no toxicology section and no environmental fate section in their template. I suggest to build that in. I have found, that most chemists appear uncomfortable with the material, be it because it reaches into other disciplines like biology, environmental sciences etc, or because of a COI if working for a producer, even though each C&En News is full of reports in that area...
I think this is a very important service, that the public looks for, and it ought to be tackled upstream, rather than in a non systematic remediative fashion, pasting a few lines here and there in grandfathered articles.
Can this please be changed? --Wuerzele (talk) 18:24, 27 August 2014 (UTC)
- The chembox contains a Hazard section, but it is often incomplete. The GHS labelling misses in most cases (as opposite to de.wikipedia). --Leyo 19:00, 27 August 2014 (UTC)hanks .
- Leyo thanks, so we agree. would you help with my request? please steer me to the hunchos working on chem templates.--Wuerzele (talk) 06:36, 31 August 2014 (UTC)
- Those hunchos are watching this. There is a bit of an issue there with respect to safety (though I agree that we can display more there) - Wikipedia should be careful with giving 'medical' advice (on the other hand, I once read that Wikipedia is really plainly used as a primary entry point for medical advice due to ease of access and the 'relative reliability' in emergency cases).
- You could easily copy the contents of Template:Chembox Hazards into a Template:Chembox Hazards/sandbox and adapt (add) fields, and similarly start creating a Template:Chembox Environment (with an own Template:Chembox Environment/sandbox). The nice thing of those sub templates is that they could be used immediately, and you can show on one or two pages the sandbox 'life' (just use 'section#=
{{Chembox Environment/sandbox|<fields>}}' instead of 'section#={{Chembox Environment|<fields>}}' and hit preview). --Dirk Beetstra T C 07:37, 31 August 2014 (UTC)- I don't think that adding fields to the chembox template is needed. It's the articles that need their hazard section being completed. For instance, not even the article ethanol contains the official GHS labelling information. In addition, the DSD classification is incomplete, since the chemical hazard symbol is missing (ref).
- What about making the GHS parameters compulsory? In cases, where neither an official GHS labelling, nor one by a chemical company is available, this fact (as opposed to cases where it exists, but is missing in the article) would need to be stated explicitly in the chembox. --Leyo 21:42, 31 August 2014 (UTC)
- Dirk Beetstra, thanks for your suggestion. I am not interested in the chembox whatsoever, since it does not fit the need- too small. I think I am being misunderstood. I am asking for mandatory description of known ecosystem hazards, and that includes human health. BTW: That has NOTHING to do with medical advice, so shouldnt be any problem; all of you hunchos hearing this?
- Leyo thanks for the suggestion-if you mean by GHS labelling info in articles, establishing 15 sections similar to the Global Harmonised System in the body of the article, we are talking the same thing. Although 15 sections sounds too much and section 4-6 (firefighting, acc release info, storage) may be a bit too much for an encyclopedia... I do agree with you, that many many (the majority ?) of WP chemicals contain no hazard/ecosystem/toxicology info. I also agree with you about unambiguously stating explicitly "no info available as of..."! as one does with N.A. in empty cells.--Wuerzele (talk) 05:07, 3 September 2014 (UTC)
- Then I am confused, you mention a template in the header of this section, which template do you mean? Also, medical advice is a bit of an extreme case, but the same goes for 'don't stuff this into your tank with piranha's' or 'don't dump this stuff in the nearest canal' .. IMHO, we should be more descriptive, 'this stuff is interfering with the whatever-biological-pathway in fish', 'this stuff accumulates in the larger predator fish which recycles it back into the human food chain in high concentrations' (I think the latter is the main reason why some compounds have that hazard-symbol). I also have problems with the 'mandatory' nature .. but there is nothing against having more information on this, indeed. Do you have an example page which has this info already? --Dirk Beetstra T C 06:06, 3 September 2014 (UTC)
- Leyo thanks, so we agree. would you help with my request? please steer me to the hunchos working on chem templates.--Wuerzele (talk) 06:36, 31 August 2014 (UTC)
- With regard to health matters, it may be worth reaching out to WikiProject Medicine; and borrowing from their well-developed policies on the inclusion and referencing of such material. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:34, 26 September 2014 (UTC)
@Wuerzele: I was not referring to a MSDS, but to labelling (example for dichlorophen; the de.wikipedia article contains this information in the chembox). --Leyo 10:17, 20 September 2014 (UTC)
ORCID
Some of you will have ORCID identifiers, as will some of the chemists you write about. ORCID is an open system of identifiers for people - particularly researchers and the authors of academic papers; but also contributors to other works, not least Wikipedia editors. ORCIDs are a bit like ISBNs for books or DOIs for papers. You can register for one, free, at http://orcid.org As well as including your ORCID in any works to which you contribute, you can include it in your user page using {{Authority control}} thus: {{Authority control|ORCID=0000-0001-5882-6823}} (that template can also include other identifies, such as VIAF, ISNI and LCCN - there's an example on my user page). ORCID identifiers can also be added to biographical articles, either directly or via Wikidata. See WP:ORCID for more. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 14:19, 1 October 2014 (UTC)
Comment on the WikiProject X proposal
Hello there! As you may already know, most WikiProjects here on Wikipedia struggle to stay active after they've been founded. I believe there is a lot of potential for WikiProjects to facilitate collaboration across subject areas, so I have submitted a grant proposal with the Wikimedia Foundation for the "WikiProject X" project. WikiProject X will study what makes WikiProjects succeed in retaining editors and then design a prototype WikiProject system that will recruit contributors to WikiProjects and help them run effectively. Please review the proposal here and leave feedback. If you have any questions, you can ask on the proposal page or leave a message on my talk page. Thank you for your time! (Also, sorry about the posting mistake earlier. If someone already moved my message to the talk page, feel free to remove this posting.) Harej (talk) 22:47, 1 October 2014 (UTC)
- On a somewhat related note (and because I was curious) some of you may be interested to learn that it will be our 12 year anniversary on the 24th December. Project Osprey (talk) 15:22, 2 October 2014 (UTC)
Biographies
I've discovered a number of "missing" biographies of chemists (lists via Wikipedia:GLAM/Royal Society of Chemistry#Articles needed). Does this project have a biography task force, or other venue where such articles are listed or discussed? Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:48, 3 October 2014 (UTC)
- I confess to being uncomfortable with a RSC-paid person creating content because it just seems like a conflict of interest, and when couple to the fact that the editor is not a chemist, seems almost pointless. But that having been said already, I looked at the list Wikipedia:GLAM/Royal Society of Chemistry#Articles needed and did not see anything particularly pressing. Mostly looked like stuff promoting RSC. The things that RSC could uniquely contribute are photographs for famous UK or FRS chemists. Ronald Sydney Nyholm, Joseph Chatt, Alan Sargeson, Bernard L. Shaw, Philip Power, and probably others need photographs. --Smokefoot (talk) 14:19, 3 October 2014 (UTC)
- I personally can't see how RSC bias could play into this. Obviously we don't want advertising copy, but I frankly think it's premature to just assume that the quality would be low just because they're getting paid by someone. It's not like we can't change the articles if they're badly written. As for the question at hand, I don't think there's a Biography taskforce here, but there is a science and academia workgroup at Wikiproject Biography.0x0077BE [talk/contrib] 16:59, 3 October 2014 (UTC)
- Re ":I personally can't see how RSC bias could play into this" Really? --Smokefoot (talk) 17:06, 3 October 2014 (UTC)
- Thank you, 0x0077BE, that's very helpful, and just what I was after. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 20:20, 3 October 2014 (UTC)
- I personally can't see how RSC bias could play into this. Obviously we don't want advertising copy, but I frankly think it's premature to just assume that the quality would be low just because they're getting paid by someone. It's not like we can't change the articles if they're badly written. As for the question at hand, I don't think there's a Biography taskforce here, but there is a science and academia workgroup at Wikiproject Biography.0x0077BE [talk/contrib] 16:59, 3 October 2014 (UTC)
Is this an actual thing? I can't say I've heard of it and the the article heavily cites the work of one man (Jonathan Williams) but perhaps I'm wrong and this isn't just another niche COI page. Project Osprey (talk) 23:34, 4 October 2014 (UTC)
- I think "borrowing hydrogen" refers heterogeneously catalyzed hydrogenations where a hydrogen donor solvent is the source of H2 that is transferred to the substrate. If my understanding is correct, the analogous process in homogenous catalysis is transfer hydrogenation, which typically uses iPrOH or formic acid as the hydrogen source. We should snoop around and sort this out. --Smokefoot (talk) 21:39, 5 October 2014 (UTC)
Could someone with a better knowledge of fluorine chemistry have a look at bifluoride. A number of unreferenced edits have been made recently. A superficial look at the literature doesn't confirm what has been stated.Axiosaurus (talk) 17:15, 4 October 2014 (UTC)
- I removed or replaced some of the more eccentric contributions from Plasmic Physics. I thought that he had agreed to consult other editors before doing this kind of stuff. Oh well.--Smokefoot (talk) 17:46, 4 October 2014 (UTC)
- Before you lay into me again, you may want to check your dates. I made no major additions since our resolution. What I did do recently, was balance the equations. Plasmic Physics (talk) 12:13, 6 October 2014 (UTC)
Royal Society of Chemistry - Wikimedian in Residence
Hi folks,
I've just started work as Wikimedian in Residence at the Royal Society of Chemistry. Over the coming year, I'll be working with RSC staff and members, to help them to improve the coverage of chemistry-related topics in Wikipedia and sister projects.
You can keep track of progress at Wikipedia:GLAM/Royal Society of Chemistry, and use the talk page if you have any questions or suggestions.
How can I and the RSC support your work to improve Wikipedia? Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 15:26, 24 September 2014 (UTC)
- I was under the impression the Wikimedian was going to have a chemistry background? V8rik (talk) 17:31, 24 September 2014 (UTC)
- Sorry to disappoint; I'm a Wikimedian in residence at a chemistry organisation, not a chemist in residence at a Wikimedia organisation. There are plenty of very knowledgeable chemists there, upon whose expertise I can call as needed. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 17:51, 24 September 2014 (UTC)
- How can you and the RSC support our work? Good question. We would like to see more active and knowledgeable editors, that is for sure. V8rik (talk) 19:44, 25 September 2014 (UTC)
- This appointment seems ridiculous - and possibly arrogant - to have a nonchemist installed in this position at the RSC. I guess we can look forward to the RSC pushing their "branding".--Smokefoot (talk) 22:31, 25 September 2014 (UTC)
- Does anyone have any use for the Wikipedian in Residence at the RSC? I mean this legitimately, because I have no idea what this position is about. Is this just a person at RSC who helps the chemists there edit wikipedia articles? Is he just going to be running training sessions or something, or is he supposed to be like helping us edit Chemistry articles himself? If the latter, I feel like his time will be more or less wasted in our very technical field, for which a single year is probably inadequate preparation. If the former, I guess I don't care all that much if he's a chemist or not. Then again, I'm not paying his salary, so it's none of my business who the RSC hires and for what purpose, just as long as he's not going to start spamming us or otherwise disruptively interfering with us. 0x0077BE [talk/contrib] 01:04, 26 September 2014 (UTC)
- I'll see if I can get the job description re-published (it was online during the recruitment phrase, but disappeared, as is usual, when the deadline passed). But in brief, the role is about outreach, training and awareness raising, not simply editing; see Wikimedian in Residence and outreach:Wikipedian in Residence for background.. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:02, 26 September 2014 (UTC)
- I've no interest in "pushing branding", nor have I been asked to do so. I've been a Wikipedian in Residence several times before, and that's never been an issue. Please judge me by my actions. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:02, 26 September 2014 (UTC)
- Does anyone have any use for the Wikipedian in Residence at the RSC? I mean this legitimately, because I have no idea what this position is about. Is this just a person at RSC who helps the chemists there edit wikipedia articles? Is he just going to be running training sessions or something, or is he supposed to be like helping us edit Chemistry articles himself? If the latter, I feel like his time will be more or less wasted in our very technical field, for which a single year is probably inadequate preparation. If the former, I guess I don't care all that much if he's a chemist or not. Then again, I'm not paying his salary, so it's none of my business who the RSC hires and for what purpose, just as long as he's not going to start spamming us or otherwise disruptively interfering with us. 0x0077BE [talk/contrib] 01:04, 26 September 2014 (UTC)
- Thank you - that's a primary objective of the initiative. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:02, 26 September 2014 (UTC)
- This appointment seems ridiculous - and possibly arrogant - to have a nonchemist installed in this position at the RSC. I guess we can look forward to the RSC pushing their "branding".--Smokefoot (talk) 22:31, 25 September 2014 (UTC)
- Welcome, Andy! Encouraging chemists at the RSC to get involved writing and improving chemistry articles would be of the most direct help to the project. I suspect one COI challenge you will face is RSC staff who want to work on RSC-related articles. Good luck in your new position. --Mark viking (talk) 03:28, 26 September 2014 (UTC)
- Thank you. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:02, 26 September 2014 (UTC)
- There is already some kind of collaboration with ChemSpider. We use ChemSpider and ChemSpider uses Wikipedia, but is this process documented anywhere, and is there any effort to get the connection up to 100% accurate? Perhaps we need a page called WP:ChemSpider where this process is described. Is there a program to provide access to RSC journals to Wikimedians prior to free access from next year? Graeme Bartlett (talk) 05:57, 26 September 2014 (UTC)
- WP:ChemSpider is a good idea; thanks. I'll raise it with the ChemSpider team next week. I'm already in discussion regarding journal access; no promises, but watch this space! Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 08:02, 26 September 2014 (UTC)
- I think you’re going to face quite a bit of this in your early days. The problem is that you’ve just been appointed here out of the blue. It is commendable for the RSC to want to get involved, but they might have thought to have talked to us about it. However, in the spirit of good faith, welcome! What you can do for us is probably tied-in with your role and like the others I would like to see some detail on what that is. More editors though, would always be welcome. This being an outreach role, what are you hoping we can do for you? Project Osprey (talk) 09:14, 26 September 2014 (UTC)
- Thank you. Like I said, I've done this before ;-) Obviously, I wasn't involved in specifying and conducting the recruitment process, but I do know that the RSC spent considerable time talking to Wikimedia UK, and to members of the editing community in the UK, including hosting an editathon (at which I volunteered as a trainer) and attending Wikimania and other events. Please see the links above for more on the WiR role. I'm already getting lots of useful ideas, such as the WP:ChemSpider suggestion above and a request on my talk page for help sourcing images. Another way members of this project can help is, as I introduce more chemists to the community of editors, to welcome, and even mentor, them. Those of you in the UK (or elsewhere - I may be travelling) might also volunteer at training or outreach events. I'll return with other ideas for collaboration as the role develops. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 09:42, 26 September 2014 (UTC)
- Andy: Do you have access to all of the journals the RSC publish? Currently there are two articles I would like to see if the content is worth while. One for example I have listed as "C. C. Addison C. D. Garner W. B. Simpson D Sutton, S. C. Wallwork Proceedings Chemical Society 367 1964" but the online index does not even seem to mention "article" names. So perhaps it is available on paper. Graeme Bartlett (talk) 08:11, 29 September 2014 (UTC)
- @Graeme Bartlett: I'm in the office tomorrow, so I'll see what I can do then, thanks. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 13:11, 1 October 2014 (UTC)
- @Graeme Bartlett: What's the other paper? Also, please mail me from an address to which I can send you a PDF. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 09:28, 2 October 2014 (UTC)
- Andy: Do you have access to all of the journals the RSC publish? Currently there are two articles I would like to see if the content is worth while. One for example I have listed as "C. C. Addison C. D. Garner W. B. Simpson D Sutton, S. C. Wallwork Proceedings Chemical Society 367 1964" but the online index does not even seem to mention "article" names. So perhaps it is available on paper. Graeme Bartlett (talk) 08:11, 29 September 2014 (UTC)
- Thank you. Like I said, I've done this before ;-) Obviously, I wasn't involved in specifying and conducting the recruitment process, but I do know that the RSC spent considerable time talking to Wikimedia UK, and to members of the editing community in the UK, including hosting an editathon (at which I volunteered as a trainer) and attending Wikimania and other events. Please see the links above for more on the WiR role. I'm already getting lots of useful ideas, such as the WP:ChemSpider suggestion above and a request on my talk page for help sourcing images. Another way members of this project can help is, as I introduce more chemists to the community of editors, to welcome, and even mentor, them. Those of you in the UK (or elsewhere - I may be travelling) might also volunteer at training or outreach events. I'll return with other ideas for collaboration as the role develops. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 09:42, 26 September 2014 (UTC)
Media
What about asking RSC for releasing some of their pictures or video clips on e.g. chemical reactions, lab equipment, etc. under a free license? Or advertising this request among interested RSC members? See also Wikipedia:WikiProject Chemistry/Image Request or Category:Wikipedia requested photographs of chemical compounds. --Leyo 12:03, 27 September 2014 (UTC)
- Thanks; I'll look into that. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 13:11, 1 October 2014 (UTC)
- I suggest that if such images are to be used, neither the article text nor caption acknowledges RSC, although maybe such could be included in the image notes. My point is to avoid the cycle where images are given to Wikipedia in return for acknowledgements. Maybe its a moot point. --Smokefoot (talk) 02:05, 2 October 2014 (UTC)
- Omitting credit where an image is used at the point an image is used is Wikipedia standard (WP:CREDITS in manual-of-style). But if an image is provided by "some source", citing that source is standard (and often mandatory) on the image-description page. That sort of detail--the distinction between making something open-license available for all to use as they see fit vs donating it for a certain use or on a certain website in exchange for "image courtesy of..." footnote--is a great think for Wikipedians in Residence to clarify and promote. DMacks (talk) 05:09, 2 October 2014 (UTC)
- I suggest that if such images are to be used, neither the article text nor caption acknowledges RSC, although maybe such could be included in the image notes. My point is to avoid the cycle where images are given to Wikipedia in return for acknowledgements. Maybe its a moot point. --Smokefoot (talk) 02:05, 2 October 2014 (UTC)
Job description
As promised, a copy of the role's job description is at:
https://www.dropbox.com/s/c9c2pmqdl36p7s0/RSC%20WiR%20Job%20description.pdf?dl=0
I'm grateful to my new colleagues for allowing it to be shared in this way, but please note that they retain copyright, and the document is not open licensed. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 11:06, 13 October 2014 (UTC)
Dear chemistry experts: Another of those old AfC drafts. Anything to this one?—Anne Delong (talk) 00:55, 12 October 2014 (UTC)
- You have pointed us to an implausible redirect, but it has been that way more than a year, so we cannot delete it any more. Is there a draft somewhere on this? Graeme Bartlett (talk) 12:08, 13 October 2014 (UTC)
- I changed the redirect to fullerene chemistry. Another possible redirect target is endohedral fullerene. --Kkmurray (talk) 14:24, 13 October 2014 (UTC)
- I'm sorry, Graeme Bartlett and Kkmurray - the link should have been Wikipedia talk:Articles for creation/Photoionization study of confined atoms inside fullerene cage, but it's been deleted now. Here's the text:
- I changed the redirect to fullerene chemistry. Another possible redirect target is endohedral fullerene. --Kkmurray (talk) 14:24, 13 October 2014 (UTC)
Dynamical Heterogeneity
Polymers are soft materials and have the property of viscoelasticity. There are two type of polymers: synthetic and biopolymers. When a polymeric liquid is cooled below its freezing temperature with avoiding crystallization then it will become a supercooled liquid and if cool further then it will become a glass. In glassy state system will fall out of equilibrium and a phase transition from polymer to glassy state is taking place.
Since polymer to glass transition have many open questions regarding relaxation time, viscosity and size of the cage etc. At low temperatures the dynamics becomes very slow(sluggish) and relaxation time increases from picosecond to second or minute or even years. In glassy state the density is not homogeneous that means particles are localized in different density distributions in space . Due to low temperature particles dynamics becomes very slow because temperature is directally proportional to kinetic energy.The particles are trapping in some local regions and rattling inside that region. These regions are called as cages in glassy polymer.
The dynamics in all these cages is different than each other so at small scale there is large number of cages in the system with respect to whole size of system. This is known as Dynamical Heterogeneity in the glassy system.
References
1.Walter Kob, Computer simulations of supercooled liquids and glasses,J. Phys.Condens. Matter 11,R85(1999).
2.Kurt Binder and Walter Kob, Glassy materials and disordered solids: An introduction to their statistical mechanics,World Scientific Publishing Co.Pte. Ltd
- I can "refund" it if it was a useful draft. Should I? —Anne Delong (talk) 10:18, 18 October 2014 (UTC)
- No need to refund, as the author went on to write the contents in Dynamical heterogeneity which is substantially the same, but improved. The title is not particularly helpful, as noted above. Graeme Bartlett (talk) 09:48, 19 October 2014 (UTC)
- Thanks for finding that. I will leave it alone. —Anne Delong (talk) 21:21, 22 October 2014 (UTC)
- No need to refund, as the author went on to write the contents in Dynamical heterogeneity which is substantially the same, but improved. The title is not particularly helpful, as noted above. Graeme Bartlett (talk) 09:48, 19 October 2014 (UTC)
- I can "refund" it if it was a useful draft. Should I? —Anne Delong (talk) 10:18, 18 October 2014 (UTC)
