Wikipedia talk:WikiProject Chemistry/Archive 31
| This is an archive of past discussions on Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 25 | ← | Archive 29 | Archive 30 | Archive 31 | Archive 32 | Archive 33 | → | Archive 35 |
Dear chemistry experts: I am not sure if this abandoned draft is about a chemistry topic, or if there is a better place to report it. Is this a notable topic, and should the page be kept instead of being deleted as a stale draft? —Anne Delong (talk) 05:59, 7 June 2014 (UTC)
- I accepted it. Graeme Bartlett (talk) 21:24, 12 June 2014 (UTC)
- Great! —Anne Delong (talk) 13:36, 25 June 2014 (UTC)
Rose oxide - isomers
The article on rose oxide states "The compound has a cis- and a trans-isomer, each with a (+)- and (−)-stereoisomer". But I see a molecule with two carbon atoms that would each independently act as a centre of chirality, and a double-bond which cannot be involved in cis-trans isomerism.
The article also contains this diagram
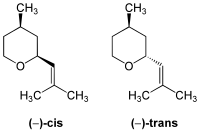 which appears to me to show something that I would not call cis/trans isomerism.
which appears to me to show something that I would not call cis/trans isomerism.
Which is confused, me or the article? Maproom (talk) 10:28, 26 June 2014 (UTC)
- The cis/trans isomerism is limited to double bonds, in this case it relates to the positioning of the groups relative to the plane of the cycle. Plasmic Physics (talk) 11:30, 26 June 2014 (UTC)
- I assume you mean "... is not limited ...". Ok, it makes sense now, thanks. Maproom (talk) 12:01, 26 June 2014 (UTC)
- Darn, for some reason I keep making a habit out of missing out a "not". Yes, that is what I meant to say. Plasmic Physics (talk) 08:01, 28 June 2014 (UTC)
Monoatomic Elements
Back in 2009, Archive 13 or 14, there was discussion on what to do with the topic of "monoatomic gold". Since about that time, I have researched in depth the topic of monoatomic elements, particularly relating to Au and the the PGE's. I found that once one sifts through all the new age chatter about "ormus" (basically magnesium hydroxide) and discredits the promotional hype of "white powder gold" product vendors, that there are scintillas of truth regarding these substances. Whether or not they are monoatomic is questionable, but they certainly appear to be allotropic forms of the elements involved. (Yes, I am aware that these metals do not have allotropes.) To further substantiate the few seemingly valid claims, I was fortunate to meet and discuss the subject with the PhD chemist who did most of the chemical extraction and purification of these substances. Enlightening to say the least. I am posting this to see if there is any interest in resurrecting this topic after five years. Your thoughts? JohnsonRick (talk) 23:48, 27 June 2014 (UTC)JohnsonRick
- If you have reliable sources then there is basically no need to ask. I struggle to see the validity of calling nano-scale assemblies of elements "allotropes" of the elements concerned. The structural "chemistry" at those scales doesn't seem comparable with what happens at the bulk scale. Sandbh (talk) 04:59, 28 June 2014 (UTC)
- Having good sources is half the requirement, it is also helpful if you are moderately fluent in the chemistry of metals. Atoms of elements are usually mentioned in the articles on those elements. A good start would be to leave a note at Talk:Gold. --Smokefoot (talk) 14:19, 28 June 2014 (UTC)
Infobox hydrogen at TFD again
{{Infobox hydrogen}} is up for deletion again. Please discuss at Wikipedia:Templates for discussion/Log/2014 June 28#Template:Infobox hydrogen. --Redrose64 (talk) 23:09, 28 June 2014 (UTC)
FYI, significant number of mislinks heading to wrong article. Discussion needed on what to do with redirect. In ictu oculi (talk) 10:49, 30 June 2014 (UTC)
Are lists like the one mentioned above of any use or significance? --Yikrazuul (talk) 09:08, 29 June 2014 (UTC)
- No. Only thing I can brainstorm would be if some special notability of their doing so (together forming a coalition or trade-group, or being together involved in some major legal or other newsworthy situation, etc.). Even then, seems likely to be unworthy of a stand-alone list article. Unless there's something notable about this group as a whole or the members of it doing the identified activity, I think at best it's just a business directory; see also Wikipedia:STANDALONE. Given the chemical involved (which might be controversial in various ways), we need to be especially careful not to make any negative connotations or implications by placing such a prominent identifier on the listed companies. DMacks (talk) 04:12, 1 July 2014 (UTC)
- I have AfD'd the article. As I state there, the opening statement could be used in the article about the compound, the list is not necessary. I find this article/principle also US-centered, this is not a 'List of 2,4-D manufacturers', this is a 'List of 2,4-D manufacturers in the US'. --Dirk Beetstra T C 10:22, 1 July 2014 (UTC)
Isomer names
The article Citral clearly states that citral is either or both two different substances, geranial and neral, which are cis-trans isomers. Yet in its infobox, the article gives a single IUPAC name, CAS number, PubChem number, ChemSpider number etc. for "citral". I had assumed that these names and numbers were meant to be identifiers. Was this naive of me? or is the infobox misleading? Maproom (talk) 12:42, 29 June 2014 (UTC)
- Citral is a mixture, so unless the isomers rapidly interconvert at standard conditions, is does not even warrant a Chembox. A table with a few simple properties at common compositions, and content describing how the properties vary with composition, should suffice. That being said, mixtures can also have identifiers, to an extent. The rule of thumb is, if you are in doubt over whether an E/Z mixture is separable, it usually is. Plasmic Physics (talk) 21:44, 29 June 2014 (UTC)
Both the mixture and pure isomers appear to have separate identifiers, that along with the common names would imply relative stability. Given time and effort the chembox could be expanded to include all the variables. However, I should point out that chembox code isn't really designed for this, I did it once for periodic acid and that involved using some of the parameters in ways they weren't intended to be.
| E/Z-citral | E-citral | Z-citral | |
|---|---|---|---|
| Cas | 5392-40-5 | 141-27-5 | 106-26-3 |
| Chemspider | 9372550 | 553578 | 558878 |
| Pubchem | 8843 | 638011 | 643779 |
On a side note, it appears that the name 'geranial' has something to do with with the Geraniums. Likewise Neral appears to be derived from Neroli. Project Osprey (talk) 23:00, 29 June 2014 (UTC)
- Geraniol has also been called “lemonol” and “rhodinol” (rose). Its structural isomers include linalool (lignum-aloes), citronellal or rhodinal, eucalyptol, lavandulol, and menthone (mint).—Odysseus1479 01:03, 30 June 2014 (UTC)
The chembox should here just be having the data for the mixture, and in the text it should link to the E and the Z compounds' articles. This chembox on Citral may become a bit minimalistic. Don't think too binary about it. --Dirk Beetstra T C 03:53, 30 June 2014 (UTC)
- Some other articles dealing with pairs of enantiomers are carvone and malic acid: each has a single chembox, giving a single IUPAC name. Would such articles be better with two chemboxes, one for each substance? (I don't think infoboxes are restricted to one per article: Klein graphs has two infoboxes, one for each graph.) Is there a reliable free online source where I can look up official names for organic chemicals? Maproom (talk) 10:25, 30 June 2014 (UTC)
- No, an article can only have one Chembox, that much is stated explicitly somewhere. And again no, there is no reliable source for official names. Plasmic Physics (talk) 10:52, 30 June 2014 (UTC)
- Agree with Beetstra: One doesnt need to get too litigious about chemboxes and isomers. Lots of compounds are available as mixtures and readers want some data on that mixture. We serve readers, period. We adapt to the chemistry, chemistry does not adapt to Wikipedia. It takes expertise to interpret data, but that is always the case. --Smokefoot (talk) 12:32, 1 July 2014 (UTC)
- No, an article can only have one Chembox, that much is stated explicitly somewhere. And again no, there is no reliable source for official names. Plasmic Physics (talk) 10:52, 30 June 2014 (UTC)
AfC Submission - Anion Exchange Resin
Got a chem-related submission at Draft:Anion Exchange Resin. Looks good at first glance to me, but I'm curious about the overlap with ion-exchange resin (if any). Is anion-exchange resin particularly notable enough to have its own article, or should it possibly be merged with ion-exchange resin? Looks like a lot of overlap coverage between the two pages, and unfortunately chemistry wasn't my forte in high school. Thanks, ~SuperHamster Talk Contribs 23:28, 30 June 2014 (UTC)
- An anion exchange resin is a type of ion exchange resin. Cation exchange resins also exist, as explained in the ion exchange resin article. In my view the draft anion exchange resin article should be rejected, as its material is already covered, better, in the ion exchange resin article. Maproom (talk) 05:59, 1 July 2014 (UTC)
- I also note that the list of "common anions" in Draft:Anion Exchange Resin, as well as having numerous errors, starts with hydride ions. You won't get hydride ions in an ion exchange resin - they react vigorously with water. Maproom (talk) 13:27, 1 July 2014 (UTC)
Noyori asymmetric hydrogenation
Can some people have a look at Talk:Noyori asymmetric hydrogenation?. Thanks! V8rik (talk) 21:32, 7 July 2014 (UTC)
- Probably should start by defining the term "Noyori asymmetric hydrogenation" because it is difficult to discuss mechanisms without knowing the process being analyzed. Not trying to be snarky here, but Noyori and co invented a lot of catalysts that hydrogenate ketones. --Smokefoot (talk) 02:36, 8 July 2014 (UTC)
Wiki Interactive Periodic Table needs to be consistent with standard periodic table.
The content of https://en.wikipedia.org/wiki/Periodic_table is okay.
What does need changing is the format of the interactive periodic table that Wiki shows for each element. For all the elements this non-standard and unusual periodic table shows at the top right corner. For example Carbon: http://en.wikipedia.org/wiki/Carbon
Why have Wiki programmers not used a periodic table format that looks like either of the first two periodic tables listed at https://en.wikipedia.org/wiki/Periodic_table ? This format is self-consistent with IUPAC, NIST, etc.
IUPAC Periodic Table. http://www.iupac.org/fileadmin/user_upload/news/IUPAC_Periodic_Table-1May13.pdf National Institute of Standards and Technology, Periodic Table. http://www.nist.gov/pml/data/images/PT-2013-Large_2.jpg
Using a non-standard periodic table is misleading and the interactive periodic table shown on Wiki for each element is incorrect.
Historical discussion of alternative, proposed, and (now considered) incorrect periodic tables is fine. But using a non-standard periodic table format for all the individual elements is not.
Wiki needs to display the commonly accepted format for the interactive periodic table shown for each element: http://en.wikipedia.org/wiki/Periodic_table#mediaviewer/File:Periodic_table_(polyatomic).svg It is the same as shown in the first two images at https://en.wikipedia.org/wiki/Periodic_table. — Preceding unsigned comment added by 128.97.138.12 (talk) 23:11, 18 June 2014 (UTC)
- It's not incorrect: it just puts the lanthanides and actinides in the main body of the periodic table, which is where they should go according to the asterisks in the main periodic table. I guess it could be a bit confusing without the element symbols, but it is equivalent to the 18-column periodic table.
- The 18-column format is widely used mostly due to space limitations on paper, but I wouldn't mind changing it to that for the purpose of recognizability (the cells would have to be slightly bigger to preserve the width, but it would become easier to click on a particular element). With the symbols included, like in the periodic table footer on each element, I think there is no problem with using a 32-column periodic table.
- Nevertheless the 18-column periodic table might be a bit confusing without the symbols: you don't know then if the convention being used is to put lanthanum or lutetium below yttrium, or none of them. With the 32-column table, you can tell between these alternatives by checking if the d-block is broken. Double sharp (talk) 02:38, 19 June 2014 (UTC)
You just single-handedly decided to ignore IUPAC, NIST, and over 100 years of periodic table history.
The placement of lanthanum (and later on actinium) under scandium and yttrium in group 3 (IIIB) has been the standard for many years; for example, the first edition of the CRC Handbook of Chemistry and Physics published 1913, page 70 titled, “Periodic Arrangement of the Elements – Mendelejeffs (Revised to 1911)” (1), and the Handbook of Chemistry published 1946 (2). This placement continues to be the standard; for example, the CRC Handbook of Chemistry and Physics (which I have access to many editions published 1968-2008) (3), the IUPAC (4), and the NIST (5).
1. CRC Handbook of Chemistry and Physics, 1st Ed., Editor David R. Lide, CRC Press, 1913, pg. 70. 2. Handbook of Chemistry, 6th Ed., Editor Norbert A. Lange, Handbook Publishers, 1946, pg. 58. 3. CRC Handbook of Chemistry and Physics, 89th Ed., Editor David R. Lide Jr., CRC Press, 2008. 4. IUPAC Periodic Table. http://www.iupac.org/fileadmin/user_upload/news/IUPAC_Periodic_Table-1May13.pdf 5. National Institute of Standards and Technology, Periodic Table. http://www.nist.gov/pml/data/images/PT-2013-Large_2.jpg
Please click on the IUPAC and NIST links. The Wiki interactive periodic table is clearly incorrect. You will not see the Wiki interactive periodic table in chemistry textbooks.
There are many problems with the Wiki interactive periodic table:
The biggest error is La, [Xe]5d1 6s2 and Ac, [Rn]6d1 7s2 are placed in the f-block even though they have no outer electrons in the f-state. As you can see from the Wiki article content La and Ac are in the first position in the d-block (Group 3) with their d1 state as they have outer electrons in common with the d-block.
Insertion of the f-block (even if done correctly which the Wiki interactive periodic table does not) is not advised for multiple reasons:
Insertion of the f-block results in these 14 elements having no group numbers (in order to maintain the original group 1-18 designations). Or one has to renumber all the groups 1-32. Halogens would then be group 31. This will never be adopted as the group numbers help explain the element properties in that group. Group 31 for the halogens (F, Cl, etc.) gives no meaning. Or keep the original group 1-18 designations and assign some other group assignment to the f-block elements. In addition to being inconsistent with most of the periodic table this would add another difficulty. Elements within a group have similar properties or trends and adjacent groups different properties. Applying this to the newly labeled groups in the f-block is not appropriate/possible.
Did it not occur to you that the Wiki interactive periodic table is causing much confusion for chemistry students who see 14 elements with no group numbers, the elements La and Ac with their outer d1 state in the f-block, etc. There is very good reason why the f-block is not "just" inserted to make the periodic table look nice.
Please change the format of the Wiki interactive periodic table to look the same as International Union of Pure and Applied Chemistry, National Institute of Standards and Technology, etc. It is the same standard periodic table as commonly accepted and shown at the beginning of https://en.wikipedia.org/wiki/Periodic_table .
- First of all, I did not single-handedly decide this, and I am not ignoring history.
- The placement of lanthanum and actinium under yttrium may have been standard for many years, but this is also changing and IUPAC chooses not to recommend a specific form (their table places neither La nor Lu under Y). There are many good arguments on both sides. And yes, Sc-Y-Lu-Lr periodic table have appeared in textbooks: one example is Wulfsberg's Inorganic Chemistry. As can be seen from "The Flyleaf Periodic Table", this usage does not appear until after 1982, when Jensen's paper "The Positions of Lanthanum (Actinium) and Lutetium (Lawrencium) in the Periodic Table" first appeared. Thereafter textbooks are about evenly split between your proposed form (14CeTh) and the current form used at the top of the infobox (14LaAc), with a few attempting to stay neutral and use IUPAC's 15LaAc.
- As Jensen says in his 2008 or 2009 paper (I think it's the former), the criteria for placing an element in the periodic table is to assign it to a block based on the orbitals its valence electrons are in, assigning it to a group based on the number of valence electrons it has available, verifying the validity of these assignments through checking trends across the block, group, and period in question, and finally verifying that the arrangement results in the elements appearing in order of increasing atomic number. For whether to place La or Lu below Y, we must go to the third criterion, as the first two don't provide a definitive answer. And indeed Jensen makes a strong case for Lu below Y in his 1982 paper, based on the properties of the elements in question and the trends that result from each choice.
- Why is the anomalous electron configuration of La and Ac absolutely prohibitive when it comes to assigning them to the f-block? By that argument thorium is also a d-block element, given its electron configuration of [Rn]6d27s2! But nobody calls it that. Lawrencium has been experimentally measured to have an electron configuration of [Rn]6f147s27p1: but does anyone call it a p-block element? And if it can be considered an f-block element with an anomalous electron configuration, why can it not be considered a d-block element with an anomalous electron configuration? I think a better way of looking at the electron configuration of La and Ac is that, like Gd and Cm, they have anomalous electron configurations to get the stability of an empty (or in the latter case half-filled) f orbital, and are really f-block elements. Finally, in lutetium, the 4f electrons are not valence electrons: therefore it does not make sense to assign it to the f-block.
- Group numbers have never been officially assigned to the f-block elements (some have tried), but is this not exactly the same situation as if they are detached from the main table? They still have no group numbers either way, and each column in the f-block still has only two elements at present. I agree with you that assigning group numbers to these columns is nonsensical due to the lack of any trends that should be present in a group, but surely inserting the f-block back into the main body doesn't change this situation.
- While group 31 for the halogens gives no meaning, neither does the IUPAC-approved group 17. The halogens all have seven valence electrons and the group number that would be best for them is surely group 7 (VIIA, VIIB, VII – whichever style you prefer): this number is far more useful than 17, for example in the (8−n) rule.
- The bottom line is that IUPAC allows both forms of the periodic table, either Sc/Y/La/Ac or Sc/Y/Lu/Lr. The long form periodic table is simply a differently laid-out version of the medium form periodic table that you will normally see, and is not as often seen merely because of the aspect ratio of paper fitting the medium form well and not the long form. This is not a constraint here, and prominent authorities such as Seaborg have preferred the long-form periodic table. Double sharp (talk) 12:29, 19 June 2014 (UTC)
IUPAC and NIST most definitely do place La and Ac under scandium and yttrium in group 3. The IUPAC table you reference clearly numbers elements 57-71 with La(57) under Y and again clearly numbers elements 89-103 with Ac(89) under La.
The vast majority of references, including IUPAC, NIST, and the Wiki page https://en.wikipedia.org/wiki/Periodic_table place La and Ac under scandium and yttrium in group 3. To state otherwise is simply not true.
The placement of lanthanum (and later on actinium) under scandium and yttrium in group 3 (IIIB) has been the standard for many years; for example, the first edition of the CRC Handbook of Chemistry and Physics published 1913, page 70 titled, “Periodic Arrangement of the Elements – Mendelejeffs (Revised to 1911)”, and the Handbook of Chemistry published 1946. This placement continues to be the standard; for example, the CRC Handbook of Chemistry and Physics, the IUPAC, and the NIST.
There is certainly room for discussion of alternative representations of the periodic table but to show the current Interactive Wiki Periodic Table and defend it as the commonly accepted form is without basis.
What is troubling is your defense of this non-standard format and then defending it on the basis that it is better than the standard format. In addition your justification comes across as informed but a good deal of it is obfuscation. Most unusual for a neutral wiki volunteer.
If IUAPC, NIST, etc., intended to place Lu and Lr in group 3 in the d-block they would have done so. The current incorrect Interactive Wiki Periodic Table places Lu and Lr in group 3 in the d-block.
You state IUPAC, NIST, etc., do not insert the f-block due to a lack of space on the printed page yet you give an online IUPAC reference. There is no restriction to IUPAC and NIST showing the inserted f-block online yet they do not (for the good reasons stated above; and it results in a gap between the group 3 elements (Sc, Y, La, Ac) and the group 4 elements. The preference, historically, has been to show gaps between blocks as a visual aid not within blocks).
This discussion is not a printed page format issue.
La, [Xe]5d1 6s2 and Ac, [Rn]6d1 7s2 are in the first position in the d-block (Group 3) because of their d1 electronic ground state and properties. The Wiki Interactive table does not show this.
Instead the Wiki Interactive periodic table shows La, [Xe]5d1 6s2 and Ac, [Rn]6d1 7s2 in an unnamed group in the f-block yet both elements have no outer electrons in the f-state (as clearly shown by their electron configurations).
Why is a wiki volunteer defending a non-standard periodic table with invalid statements?
Using your reasoning Cu, [Ar]3d10 4s1 and Zn, [Ar]3d10 4s2 would be moved to the s-block.
Lu, [Xe]4f14 5d1 6s2 and Lr, [Rn]5f14 7s2 7p1 are best left in the f-block because both elements have outer electrons in the f-state (as clearly shown by their electron configurations).
The Wiki Interactive Periodic Table format must be self-consistent with the format of other online periodic tables by established scientific bodies such as IUPAC and NIST. — Preceding unsigned comment added by 100.32.69.62 (talk) 02:52, 20 June 2014 (UTC)
- No, they don't. If they had wanted to unequivocally dictate that the composition of group 3 is Sc/Y/La/Ac, they would have done so by putting La and Ac in the main body, followed by a note leading to the lanthanides and actinides at the bottom of the periodic table. So group 3 would look like Sc/Y/La/Ac: followed by a space with boxes reading (58-71) and (90-103): and then group 4 (Ti/Zr/Hf/Rf). This is not what IUPAC does: they sidestep the issue. How do you know they didn't literally mean that all the lanthanides and actinides fitted under Sc and Y?
- The online IUPAC reference is the one you yourself mentioned, and that is why I commented on it. The medium period layout probably stemmed from page limitations IIRC. While it is still commonly used even elsewhere (I would imagine because the Web gives you freedom to use any aspect ratio you'd like, and it's easier to use the traditional format), now that the restriction is not present, it is absolutely OK to use an equivalent form. In fact we don't always use the long form: we just use it here because the medium form won't fit the limited width as well. This isn't even something like using the Janet periodic table (putting He over Be) or some of those formats that put Be and Mg over Zn, etc. All the elements are in the same locations as the medium table implies they are. As for the La/Lu issue, different forms of the medium table imply differently on this issue.
- No, La and Ac are not d-block elements, as I have said earlier: they are f-block elements with anomalous electronic configurations. Or do you think the electron configurations of almost all the f-block elements are anomalous, because most of them don't have the 5d1/6d1 electron that was filled in La and Ac? Even their properties indicate this: the trends you get when plotting Sc-Y-Lu resemble the d-block groups very closely, unlike the trends you get when plotting Sc-Y-La. See Jensen's 1982 article for some graphs. Putting La and Ac in the d-block means that in group 3 alone (and in none of the other d-block groups), there is no addition of the filled 4f14 subshell when going down from period 5 to period 6.
- Please read carefully. I never said that thorium was a d-block element: I merely pointed out that by your argument it should be one because of its [Rn]6d27s2 electron configuration. Naturally I disagree with that, and hence I think that Cu and Zn should be handled the same way: they are d-block elements, although Cu has an anomalous [Ar]3d104s1 electron configuration.
- Finally, if you're using the standard definition of what an outer electron is (I'm assuming a valence electron), the f electrons in Lu and Lr are not outer electrons as they don't participate in chemical bonding: the 5d1 and 6s2 electrons of Lu are lost to form the Lu3+ ion, with the 4f electrons firmly part of the core and not participating in chemical reactions (the 4f shell being well-localized near the atomic nucleus). Just like the next elements (Hf and Rf) the s and d electrons (p instead for Lr) are the valence electrons, and hence these elements are in the d-block. Double sharp (talk) 05:01, 21 June 2014 (UTC)
- (P.S. I wouldn't oppose changing the long table to a medium table if there was a clear consensus to do so, but the issue has been raised several times and there never seemed to be one each time. What I personally cannot support is following Sc/Y/La/Ac when arguments based on the properties of the elements oppose this format and IUPAC and NIST do not use it. Sc/Y/*/** would be the best, remaining neutral, but that is tricky to do with the long table without grotesquely stretching the Sc and Y cells: hence for the long table Sc/Y/Lu/Lr is probably best.) Double sharp (talk) 05:09, 21 June 2014 (UTC)
- re IP. An introduction. Here I'll write about the graphical presentation, which is secondary to the facts we actually want to show. A PT graph follows scientific statements. I have shaped many of the PT's here at enwiki, usually following an outcome of a discussion at WT:ELEMENTS. This is about the drawing of PTs; the actual facts the PT shows is beyond my area of knowledge (I can read & follow those PT content discussions, but not make new statements). I note that similar discussions exist for: which are the metalloids?, are group 12 elements a post-transisiton metals?, what to name those post-transition metals after all?, and —less hot— the placement of hydrogen (H). At this point, please note that all these are not internal wiki issues, but they reflect topics as they exist in the scientific world.
- In most or all PTs at en:wikipedia, when we show a positioning it is that group 3 is Sc/Y/Lu/Lr. This is usually a 32-column PT. In 18-column PTs, we show group 3 and the asterisks being ambiguous (as in this PT).
- Let me split the topic. IP conflates the PT graph with statements of element positioning. The group 3/f-block/La&Ac/Lu&Lr/ topic as scientific statements and facts is independent of and prior to any presentation form. The PT graphic should simply represent whatever the outcome is, maybe even showing an ambiguity in the theories. From this I note that IP says (my words): "the 18-column form is better because it shows the facts as they are correct". Whether these statements are correct and which statements to use in our PTs, I'll leave to others for now (Double sharp addresses this above). Such a scintific debate can very well be described in an article or article section, for example in group 3.
- 18-column variants. Contrary to what IP seems to think, there is no single 18-column PT standard with regard to this issue. We could also show an 18-column PT with Lu/Lr in group 3 explicitly; a statement IP opposes. So "the" 18-column format does not say anything about these placements.
- Graphic clarity. For any graphic presentation, I require that it is unambiguous in what it conveys. (If we want to show the dual options for hydrogen, then we must find a way to show that scientific ambiguity graphically unambiguous!). We should not ever put graphic ambiguity on the readers screen. In this topic of the outplaced set of elements, it must be clear how to position that satellite elements back into the periodic table. This cut-and-paste exercise may not introduce any unclarities or questions, either when taking them out to below or putting them back in. I repeat: no graphic ambiguity.
- The solution IP proposes make use of the graphic ambiguity of the 18-column PT: that 18-column variant graphically hides the group 3 discussion, it simply does not show any facts or discussion. But in a 32-column presentation we cannot hide it. Again, I refuse to use an 18-column PT because of this 'advantage'. It leaves the reader with questions.
- 18-column PTs questioned. Actually, our commonly used PT (such as the first one here) does have an ambiguity. The asterisks show the replacement positions of the two elements rows clearly (I hope). But when I cut-and-paste 15 elements in there (say the LN), I have no clue as to what happens with that group 3 column. Are those 15 elements squeezed into one cell (a sin in PT structuring, but graphically suggested)? Are Sc/Y glued to group 2 or to group 4? Are Sc and Y cells somehow stretched to span the whole area between group 2 and group 4? This is the graphical imperfection in the PT we use. (btw, all of this could be clarified by adding a gap column between groups 2–3 [1] or between groups 3–4. This requires a choice of group 3 completion as is our topic here).
- The IUPAC PT that IP has linked to [2] is a graphically a rejectable form and a horror. Those vertical dashed lines making a vertical connection in group 3: what does that say? Are La and Ac really below there in group 3 (suggesting some extra periods)? Or if I copy-paste elements La and Ac into periods 6 and 7, what to do with the remaining 2×14 elements? Are these Desert Island elements? And still the gap question for periods 4 and 5 is not clarified. IUPAC should never have shown this graph to the world.
- Some more bad graphics (what does the graph say about the group 3 area? How to cut&paste LN/AN?): [3], [4], [5] (ouch), [6], [7], [8],
- Also, I find it a bit weird to use "historical" as an argument. The PT structure has always been adjusted in its 150+ years. Why use a form 100 years old when the LN/AN were not even recognised?
- Conclusion. The topic IP raises should be split into 1. what facts to show (periodic table scientific), and 2. then follows: how to show that outcome unambiguously in any PT. I am amxious to learn what facts we should show. If someone can show it in a 32-column PT, we can graph that into an 18-column for sure. -DePiep (talk) 12:28, 22 June 2014 (UTC)
- Yes indeed, the historical argument is quite weak: if we're going to use this argument, why not go back to the beginning of the story and use Mendeleev's original 8-column short-form periodic table? It's still very popular in the CIS even now. Double sharp (talk) 13:21, 22 June 2014 (UTC)
- re IP: The Wiki Interactive Periodic Table format must be self-consistent with the format of other online periodic tables by established scientific bodies such as IUPAC and NIST
- NIST? Never heard of, never met a link! That's the "National institute of Standards and Technology" then (which Nation??). But thanks. Now, for the moment, about their "standards". The NIST PT graph (nice!) still does not resolve the group 3 area: when I insert the LN/AN elements in there as they show, what or where or how are Sc/Y to go?
- Your IUPAC PT, as I wrote above, has an illegible graph. I wrote: It is a graphically a rejectable form and a horror. Those vertical dashed lines making a vertical connection in group 3: what does that say? Are La and Ac really below there in group 3 (suggesting some extra periods)? Or if I copy-paste elements La and Ac into periods 6 and 7, what to do with the remaining 2×14 elements? Are these Desert Island elements? And still the gap question for periods 4 and 5 is not clarified. IUPAC should never have shown this graph to the world.
- Please, please show me your 32-column PT. -DePiep (talk) 01:40, 24 June 2014 (UTC)
- I think what (s)he wants is a 32-column table where La and Ac are in group 3, and the remaining 2×14 elements are spliced between La and Hf, like this one. (That old revision moreover gives the first 4 hypothetical period 8 elements, to show where they would fit. But it breaks all the blocks except the p-block.) Double sharp (talk) 15:14, 24 June 2014 (UTC)
- Yes indeed, the historical argument is quite weak: if we're going to use this argument, why not go back to the beginning of the story and use Mendeleev's original 8-column short-form periodic table? It's still very popular in the CIS even now. Double sharp (talk) 13:21, 22 June 2014 (UTC)
- (P.S. I wouldn't oppose changing the long table to a medium table if there was a clear consensus to do so, but the issue has been raised several times and there never seemed to be one each time. What I personally cannot support is following Sc/Y/La/Ac when arguments based on the properties of the elements oppose this format and IUPAC and NIST do not use it. Sc/Y/*/** would be the best, remaining neutral, but that is tricky to do with the long table without grotesquely stretching the Sc and Y cells: hence for the long table Sc/Y/Lu/Lr is probably best.) Double sharp (talk) 05:09, 21 June 2014 (UTC)
Yes IUPAC side-steps the issue of the ‘best’ periodic table format/presentation for many good reasons. Some of which have been discussed above (f-block elements have no group numbers; “Elements within a group have similar properties or trends and adjacent groups different properties. Applying this to the newly labeled groups in the f-block is not appropriate/possible.”; visual gap within a block; etc.)
However the IUPAC has publications which make clear the group 3 elements are Sc, Y, La, Ac. For example the 2014 publication:
IUPAC-NIST Solubility Data Series. 100. Rare Earth Metal Fluorides in Water and Aqueous Systems. Part 1. Scandium Group (Sc, Y, La)
They do this because of over 100 years of placing La in group 3. See detailed comments and references above.
The group 3 d-block elements are: Sc, [Ar]3d1 4s2 Y, [Kr]4d1 5s2 La, [Xe]5d1 6s2 Ac, [Rn]6d1 7s2
The Wiki Interactive Periodic Table places La, [Xe]5d1 6s2 and Ac, [Rn]6d1 7s2 in the f-block. This creates many problems and is a step backwards.
If we start moving elements around because of ‘anomalous’ electronic configurations then there are many elements that need moving. One cannot selectively justify one argument for moving some elements and not apply the same to other elements.
If one applies your reasoning that Lu and Lr do not belong in the f-block because, “the 4f electrons firmly part of the core and not participating in chemical reactions”, then that means the entire 4f elements/row don’t belong in the f-block!
Lu, [Xe]4f14 5d1 6s2 and Lr, [Rn]5f14 7s2 7p1 are best left in the f-block because both elements have outer electrons in the f-state (as clearly shown by their electron configurations). This is commonly accepted practice, e.g., http://www.rsc.org/periodic-table/element/71/lutetium
Keeping Lu, [Xe]4f14 5d1 6s2 and Lr, [Rn]5f14 7s2 7p1 in the f-block makes sense for several reasons. Even though the tightly held 4f electrons in the lanthanides do not participate in chemical reactions the 4f electrons do play a role in their properties. It is for this reason there is active research in lanthanide-doped semiconductors with small amounts of lanthanide ions and the important role of their 4f transitions. For example the publication titled, “Synthesis, Structure, and Molecular Orbital Studies of Yttrium, Erbium, and Lutetium Complexes Bearing Pyrazolato Ligands: Development of a New Class of Precursors for Doping Semiconductors”.
The Wiki Interactive Periodic Table incorrectly removes Lu, [Xe]4f14 5d1 6s2 from the f-block and incorrectly places La, [Xe]5d1 6s2 in the f-block, yet there is obviously no way the 4f electrons in La can play any role in any process because it has no 4f electrons!
For all the detailed defense of the unusual Wiki Interactive Periodic Table it is surprising that there is any confusion as to what standard format I am referring to. As already stated many times the format of the first two periodic tables listed at https://en.wikipedia.org/wiki/Periodic_table are fine to use for the Wiki Interactive Periodic Table, as are the IUPAC periodic table http://www.iupac.org/fileadmin/user_upload/news/IUPAC_Periodic_Table-1May13.pdf, the National Institute of Standards and Technology periodic table http://www.nist.gov/pml/data/images/PT-2013-Large_2.jpg, or any of these:
Although I would recommend the Wiki Interactive Periodic Table uses the IUPAC format.
Please note I am not asking or suggesting that the discussion of these problems not be present in Wiki articles of which there are 100’s of Wiki pages on the periodic table and the individual elements.
However the current Wiki Interactive Periodic Table is definitely a misrepresentation of what is the commonly accepted format (see IUPAC, NIST, https://en.wikipedia.org/wiki/Periodic_table).
The current Wiki Interactive Periodic Table incorrectly places La, [Xe]5d1 6s2 and Ac, [Rn]6d1 7s2 in the f-block and incorrectly places Lu, [Xe]4f14 5d1 6s2 and Lr, [Rn]5f14 7s2 7p1 in the d-block. The word “incorrectly” is used for the many reasons discussed here and above. — Preceding unsigned comment added by 128.97.138.12 (talk) 23:26, 24 June 2014 (UTC)
- No, it doesn't mean that the entire row doesn't belong in the f-block. To take the example of Yb (although it will really work for any lanthanide except La and Gd), [Xe]4f146s2, two 6s and one 4f electron are ionized to make the tripositive ytterbium cation with electron configuration [Xe]4f13. Clearly the 4f electron is active here. Indeed this holds for every lanthanide except La and Gd, which have anomalous electron configurations, as I have said many times. (Ce's 4f electron is ionized when it is oxidized to Ce4+.) And this says nothing about other elements: many also have anomalous electron configurations, like Cr, Cu, Pd, etc. Is it so hard to believe that La and Ac are also like this? And why do your arguments suggest that Lr's [Rn]5f147s27p1 electron configuration prohibits it from being a d-block element, but has absolutely no bearing on whether it is an f-block element?
- You don't have to move any other elements. Cu is in group 11, as that is where it would be if it had the expected [Ar]3d94s2 electron configuration. Thus La is the first f-block element, as that is where it would be if it had the expected [Xe]4f16s1 electron configuration. Lu presents no argument with its [Xe]4f145d16s2: it has the right configuration to be under Y. After all Hf with [Xe]4f145d26s2 is under Zr.
- I can handle a visual gap in the d-block, and you don't even need one in Sc/Y/La/Ac if the cells read Sc/Y/La*/Ac** or similar. And simply removing the f-block from the main table doesn't solve the problem of the f-block columns: they are still columns, and the outermost ones are still adjacent to groups 2, 3 or 4 (depending on format), because that is where the asterisks seem to be telling me to place them. Double sharp (talk) 02:44, 25 June 2014 (UTC)
On the basis that Lu 4f electrons do not participate in bonding you stated it as a reason not to include Lu in the f-block. The 4f electrons for all the Lanthanides do not participate in bonding, and we are in agreement this is not a reason to remove them from the f-block. This shows the importance of electron configurations and where elements are placed, and Lu [Xe]4f14 5d1 6s2 which is nearly always Lu3+ [Xe]4f14 has outer 4f electrons that can play a role as discussed above in the example of lanthanide-doped semiconductors.
That is why their publication is titled, “Synthesis, Structure, and Molecular Orbital Studies of Yttrium, Erbium, and Lutetium Complexes Bearing Pyrazolato Ligands: Development of a New Class of Precursors for Doping Semiconductors”.
It is not titled, “Synthesis, Structure, and Molecular Orbital Studies of Yttrium, Erbium, and Lanthanum Complexes Bearing Pyrazolato Ligands: Development of a New Class of Precursors for Doping Semiconductors”, for the obvious reason that La, [Xe]5d1 6s2 has no 4f electrons.
I am also in full agreement with your statement, “I can handle a visual gap in the d-block, and you don't even need one in Sc/Y/La/Ac if the cells read Sc/Y/La*/Ac** or similar.” This what the IUPAC, NIST and the above examples do. When will the Wiki Interactive Periodic Table show this format? — Preceding unsigned comment added by 128.97.138.12 (talk) 19:32, 26 June 2014 (UTC)
- As I have said many times above, no, they don't. They use Sc/Y/*/**. Double sharp (talk) 14:13, 1 July 2014 (UTC)
- IUPAC doesn't have a position on the composition of group 3. A number of chemists in the 1920's and 30's assigned Lu rather than La to group 3 on the basis that the chemical properties of Y, and Sc to a lesser extent, were closer to Lu. That La and Ac are sometimes shown as group 3 members appears to have originated in the 1940s based on electronic configurations and the concept of the differentiating electron. As noted, arguments as to the composition of group 3 should turn upon more than the single concept of a differentiating electron. There remains a reasonable body of physics and chemistry-based evidence favouring the assignment of Lu and Lr to group 3. Eric Scerri has recently presented arguments [9], including those based on the construction of the 32-column form of the periodic table, supporting the assignment of Lu and Lr to group 3. IUPAC have since asked him to form a working group with a view to making this change official. See also Jensen's 2009 commentary on this question, here. Sandbh (talk) 11:36, 25 June 2014 (UTC)
IUPAC has for many years not wanted to force this issue because there is no one correct periodic table format when it comes to the f-block. But what is clear is the preferred representation as shown by: the first two periodic tables listed at https://en.wikipedia.org/wiki/Periodic_table the IUPAC periodic table http://www.iupac.org/fileadmin/user_upload/news/IUPAC_Periodic_Table-1May13.pdf the National Institute of Standards and Technology periodic table http://www.nist.gov/pml/data/images/PT-2013-Large_2.jpg
And 2014 publications such as “IUPAC-NIST Solubility Data Series. 100. Rare Earth Metal Fluorides in Water and Aqueous Systems. Part 1. Scandium Group (Sc, Y, La)” are unambiguous.
- I agree with you that the 18-column form is prevalent. I don't like the fact that the lanthanides and actinides are relegated to the status of footnotes, nor do I like the fact that the IUPAC table shows 15 lanthanides, rather than 14, due to indecisiveness as to the composition of group 3. Since the 18 column-form is prevalent it's reasonable to show this as the Wikipedia periodic table. Given the shortcomings of this form I think having the 32-column form in element infoboxes is a reasonable way of accommodating (a) prevalence and (b) showing what's really going on. Sandbh (talk) 00:11, 29 June 2014 (UTC)
“That La and Ac are sometimes shown as group 3 members appears to have originated in the 1940s based on electronic configurations and the concept of the differentiating electron.” Sorry but this 100% incorrect.
Lanthanum was placed under scandium and yttrium in group 3 over 100 years ago. See the first edition of the CRC Handbook of Chemistry and Physics published 1913, page 70 titled, “Periodic Arrangement of the Elements – Mendelejeffs (Revised to 1911)”.
Lanthanum was placed under scandium and yttrium in group 3 in the major chemistry reference books and still is. Examples are the Handbook of Chemistry published 1946 and decades of the CRC Handbook of Chemistry and Physics.
- I should've been clearer. IMO, since about the 1940s, authors who show La in group 3 have done so explicitly on the basis of the electron configuration argument; or tacitly on the basis of how other authors have depicted their PTs. In both cases, strong physical and chemical arguments for placing Lu in group 3 have been discounted or overlooked. Sandbh (talk) 00:11, 29 June 2014 (UTC)
The Scerri link refers to Jensen’s early paper and Jensen’s paper is selective in omitting the reasons why La and Ac were and are placed in group 3.
- He discusses the relevance of electron configurations well enough, I think. And he gives the physical and chemical arguments for his case. Sandbh (talk) 00:11, 29 June 2014 (UTC)
You are restating what I wrote, he did provide the physical and chemical arguments for his case. He did not discuss the physical and chemical arguments for why La and Ac were and are placed in group 3 and the implications of moving La and Ac to the f-block (which are discussed above).
One has to look at the pros, cons, and implications of placing elements in the periodic table or changing their positions.
In addition the f-block, as discussed in above previous posts, are not groups like the other periodic table groups and this is a reason not to insert them or one ends up with unnumbered/unnamed/non-groups being presented as groups. Hence the IUPAC presentation which they are unlikely to change.
- See here, for an alternative view, including that of Eric Scerri. Sandbh (talk) 00:11, 29 June 2014 (UTC)
However whatever changes are proposed the Wiki Interactive Periodic Table should be reflecting what is the preferred periodic table presentation as shown by: the first two periodic tables listed at https://en.wikipedia.org/wiki/Periodic_table the IUPAC periodic table http://www.iupac.org/fileadmin/user_upload/news/IUPAC_Periodic_Table-1May13.pdf the National Institute of Standards and Technology periodic table http://www.nist.gov/pml/data/images/PT-2013-Large_2.jpg etc. (see links in previous post).
(As an aside where was Jensen’s emotional commentary published? Jensen has formatted it to look like a journal publication but I could not find it.) — Preceding unsigned comment added by 128.97.138.12 (talk) 20:27, 26 June 2014 (UTC)
- I understand it was published in a condensed form in the Journal of Chemical Education. I further understand that his commentary may appear to be somewhat emotional as he perceived that he had been subjected to an "ad hominen" attack, without warning. PS: I collapsed your earlier links as they display poorly on my ipad. Sandbh (talk) 00:11, 29 June 2014 (UTC)
Most unusual to show a paper in journal format, and have it referenced by others as a publication, when it is not a journal publication.
- Here is the condensed form Sandbh refers to. Double sharp (talk) 15:14, 8 July 2014 (UTC)
Support for Chemical table files
As of now, images of structural formulas have to be created using third party software and converting the output to SVG or PNG. With MolHandler we aim for a solution capable accepting and rendering chemical markup files and providing a web-interface for easily creating, modifying and re-mixing formula files. This does not only make re-using existing structures easier and simplifies creation of structures, moreover it allows Wikis to adopt a unified style for rendering these structures, makes structures searchable (sub-structure search) allows pulling, pushing and verifying data from big databases like ChemSpider and PubChem. In the future we plan to enable support for spectra and more sophisticated file formats to have at least some minimum support forchemistry-related Wiki-works.
I am currently looking for features you would find helpful as well as your opinion of what is needed to deploy MolHandler to Wikipedia and therefore created a test wiki at which you can create user accounts (and do everything you ever wanted to do). A non-exhaustive list of features is available for raking by drag&drop. Or just write here what you at least want, what you would like to see soon and what is less important to you.
- TLDR
- If you want to upload MOL or RXN files instead of SVGs and PNGs in future, go to http://mol.wmflabs.org/, test and say, "YES to MolHandler"!
-- Rillke (talk) 13:00, 8 July 2014 (UTC)
- This sounds promising. To report issues at bugzilla is too much trouble. Can we have a talk page on the mol Wiki to discuss the experiences? Graeme Bartlett (talk) 23:12, 8 July 2014 (UTC)
- Of course. Thanks for your error report. Also note that I may delete stuff from MolWiki and migrate it to Bugzilla or Phabricator to be able to keep track of everything (Mailinglist threads and RfCs on other Wikis are running as well, in case you are wondering why). -- Rillke (talk) 11:14, 9 July 2014 (UTC)
Dear chemists: This old AfC submission will soon be deleted as a stale draft. Is this a notable chemistry topic? The text seems polished in a way that makes me wonder if some of it has been taken from a book or journal article. —Anne Delong (talk) 20:41, 8 July 2014 (UTC)
- Organostannane addition possibly deals with the same reaction V8rik (talk) 21:59, 8 July 2014 (UTC)
- Reasons given for rejection dubious. No wonder we rarely get to see new chemistry content. V8rik (talk) 22:04, 8 July 2014 (UTC)
- Allylation is only a redirect to a small entry in Allyl, where a single asymmetric methodology is mentioned (but not Keck's). I could easily see having a real Asymmetric allylation article (there are multiple variants) and/or Keck allylation (it seems to be a standard Name reaction). I'm not sure the intersection of those two is sufficiently notable for a stand-alone article and this AfC does seem to need a lot of work to focus on its topic, but it surely seems like a viable starting point even if it eventually would get merged or distributed somewhere else. I agree with V8rik's thoughts about the rejection itself and a possible effect of it. DMacks (talk) 22:28, 8 July 2014 (UTC)
- I agree with V8rik that the reasons for rejection of this draft are dubious. It would be a shame for this article to be deleted, so I have moved it to asymmetric Keck allylation. As DMacks notes, it may be better as a part of broader topic such as allylation. Please feel free to rework, merge, etc. the content as you see fit. But for now, at least it is saved from deletion. -- Ed (Edgar181) 22:36, 8 July 2014 (UTC)
- To find other articles about chemistry that are sitting in Articles for Creation, try typing the word chemistry into this search box:
 | Find articles in Articles for Creation
|  |
Those with a grey box at the top have never been submitted; those with a yellow box are currently waiting review; those with a pink box have been declined. Those with a darker pink bar across the middle of the box are abandoned and facing deletion, but this deletion is delayed for six months if even one edit is made. Please don't edit ones that you think should be deleted, but if you see an abandoned one that looks viable, feel free to fix it up. —Anne Delong (talk) 04:11, 9 July 2014 (UTC)
- Anne has done a terrific job bringing these new articles to our attention and asking for comments, which many of us have made. Looking through the 150 more recent "articles for creation," almost all are requests for biographies. The following is a list of chemical topics, several of which have already gotten comments thanks to Anne's efforts.
- Wikipedia talk:Articles for creation/Proline organocatalysis
- Wikipedia talk:Articles for creation/Carbohydrate chemistry
- Wikipedia talk:Articles for creation/Edwards Equation
- Wikipedia talk:Articles for creation/Aluminum in food
- Wikipedia talk:Articles for creation/aza-Cope rearrangement
- Wikipedia talk:Articles for creation/Phenacenes
- Wikipedia talk:Articles for creation/Hantz reactions
- Wikipedia talk:Articles for creation/Azaspiracid
--Smokefoot (talk) 11:44, 9 July 2014 (UTC)
- Thanks, Smokefoot, for doing the sifting. I notice that this one has had its AfC template removed, and is just sitting there going nowhere, so maybe someone would like to take a look at it. —Anne Delong (talk) 12:48, 9 July 2014 (UTC)
- Looks like The aza-Cope rearrangements is a live page created by the same editor just after he stopped doing extensive work on Wikipedia talk:Articles for creation/aza-Cope rearrangement ("this one"), so the draft can probably be deleted. As with many presumable student works, this looks like it needs a bunch of work to be something more usable by non-experts, and general MOS. DMacks (talk) 14:19, 9 July 2014 (UTC)
- I moved The aza-Cope rearrangements to the title aza-Cope rearrangement, merged the editing history from the AFC, and redirected the AFC to the article. -- Ed (Edgar181) 17:51, 9 July 2014 (UTC)
- Hi Anne, thanks for the effort and thanks for mobilizing the chemistry community V8rik (talk) 16:54, 9 July 2014 (UTC)
- I moved Phenacene and Azaspiracid to mainspace and did a bit of cleanup on each. -- Ed (Edgar181) 17:51, 9 July 2014 (UTC)
- Hey, this is great! Watch for a similar thread in the future about the ones in the new "Draft" namespace. —Anne Delong (talk) 01:03, 10 July 2014 (UTC)
There is a chemistry-related AFD that may interest members of this WikiProject: Wikipedia:Articles for deletion/Cyclomethane. ChemNerd (talk) 11:49, 31 July 2014 (UTC)
Nomination for deletion of Template:Material properties (thermodynamics)
![]() Template:Material properties (thermodynamics) has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. DH85868993 (talk) 11:13, 14 August 2014 (UTC)
Template:Material properties (thermodynamics) has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. DH85868993 (talk) 11:13, 14 August 2014 (UTC)
Editor conflict at Steroid
@User:Boghog, please state your argument here, that the steroids article should be moved away from IUPAC and NLM Mesh inclusion of secosteroids, to a different definition. Note to readers, this is raised here because Bohog has strongly edited current text at the foundational Secosteroid stub, essentially reverting my earlier work, and in so doing changed the direction of the Secosteroid and ultimately the Steroid article — this despite (from Talk at Steroid) knowing he did not have consensus. The Steroid article cannot both proceed with, and without the seco-, nor-, and homosteroid classes included, and the opening lede secosteroid definition at Secosteroid is the first step in redefining the content of the Steroid article. Hence, the lede definition question is a central to the direction of the Steroid article. It also has implications for nor- and homosteroids, and aza-/oxa and other categories included in IUPAC, Dictionary of Chemistry, Nat Prod Reports, and other classic sources not sympathetic with the "secosteroids not steroids" initiative. The underlying issue appears -- based on Talk at steroid -- to be that Boghog only wants only tetracyclic structures included in the Steroid article.
Boghog, discussion to you. And I ask you again, belay your impatience to change the Secosteroid and Steroid articles immediately (because you are angry with me over the nat products article, or whatever). Please do not revert and disrupt the Secosteroids and Steroids articles as they are (regarding Secosteroid inclusion), until we can reach consensus, including hearing voices from this and other projects. (I have posted a brief notice at Pharmacology; feel free to post the question in other venues as well.) Le Prof Leprof 7272 (talk) 23:23, 15 July 2014 (UTC)
- Do I now see it correctly, Leprof 7272, that you are busy changing the articles over (without prior discussion), and that Boghog has reverted that because they disagree with that? --Dirk Beetstra T C 03:32, 16 July 2014 (UTC)
- Dirk, in separate, full answer to your question, the backstory is as follows. When I edited the Secosteroid article lede back in early May—an article which had see no attention from anyone in many, many months—I did so because the lede did not reflect the definitions appearing in the IUPAC, IUBMB, NLM, and more specific journal (Nat Prod Reports, J Nat Prod, Steroids) categories and coverages. These coverages speak of the subcategories of steroids (seco, nor, homo, hetero) as just that, subcategories of steroids, and not as compounds similar to them. I therefore made the change in May. There was really no one there to discuss this with, but, if you look at Steroid Talk, [10], you will see that every significant change I made there was accompanied by a Talk discussion section, asking input at times, or at least explaining the course of the editing. Note, I was brought to the Secosteroid article after beginning to add seco-, homo-, and nor- material to the Steroids site, and realizing there was a disparity in definitions between the two sites (see this material, [11]).
- The edit to return the definition to "similar to" was then made mid July by Boghog, immediately following a discussion in Talk at Steroid. It seems clear from that discussion and his subsequent change of the Secosteroid language to "is similar", that the eventual aim is to move Steroid from covering all these subcategories, to just covering the largest tetracyclic 6-6-6-5 tetracyclic structural class. As I argue at Secosteroid talk, this defied all of the solidly sourced, broadly accepted categorizations and source coverages indicated above. If you want something on azasteroids, you go to steroid journals. If you want to name a secosteroid, it is in the IUPAC/IUBMB nomenclature article for steroids. If you are curious about a particular nor- or homo- or seco- steroid, you can find it in the venerable 2 vol Dictionary of Steroids. That some online medical dictionaries use language indicating "derived from" is immaterial because subcategories of steroids routinely derive from other subcategories. That other online medical sources use "is similar" language is worth mentioning, but should not trump IUPAC, IUBMB, and standard usage where seco-steroids are a subcategory in the whole of the steroid category (alongside homo-, nor-, hetero-, etc.). That about.com says otherwise does not sway me, nor does attempting to interpret the NLM definition for steroids to exclude the secosteroids persuade, when the NLM Mesh overtly lists secosteroids as a subcategory of steroids. As I state at Secosteroid Talk, the desire for pedagogic simplicity and a few stray web dictionary definitions cannot trump the preponderance of usage in the major secondary sources and society and nomenclature tomes.
- This is an important guiding question, hence the revert (something I have only done a handful of times here), and hence the request for broad participation. The issue is which understanding will guide the development of the Steroid article (which currently parallels the definition in place, [12], prior to Boghog's edit of this week). Le Prof Leprof 7272 (talk) 06:52, 16 July 2014 (UTC)
- The proper place is to discuss this in on the secosteroid talk page, not here. In answer to Dirk's question, an older version of the article stated "a secosteroid is a molecule similar to a steroid but with a 'broken' ring". Leprof changed that to a secosteroid is a subclass of steroids. I subsequently restored the introductory sentence and added "Secosteroids are variously defined as a subclass of steroids or derived from steroids" with reliable sources to back both definitions. Then Leprof reverted my changes. There is a related discussion here. Boghog (talk) 03:56, 16 July 2014 (UTC)
- Boghog has requested that the discussion take place here: [13], and I do not disagree. Le Prof Leprof 7272 (talk) 06:52, 16 July 2014 (UTC)
- If the discussion started on that page then it should continue on that page. Otherwise it becomes fragmented and the eventual conclusion may get lost in the archives of this page... and if that happens we may end up having the same discussion some years later, when someone finds a half finished conversation on the article talk page and tried to finish it. Such things have happened before. Project Osprey (talk) 08:24, 16 July 2014 (UTC)
Questionable category
I've found a newly created category that does not have a well-defined candidate definition, and furthermore, its usefulness is not evident. Category:Bulk chemicals. Perhaps, it is a CFD? Plasmic Physics (talk) 09:13, 17 June 2014 (UTC)
- "Bulk" is certainly vague, but I don't see a problem with the concept of categorizing chemicals which have high commercial use. Maybe the category could be defined (and/or renamed) along the lines of High production volume chemicals which has an unambiguous definition. -- Ed (Edgar181) 12:48, 17 June 2014 (UTC)
- I think commodity chemicals is a less-vague synonym for bulk chemicals. Antony–22 (talk⁄contribs) 17:49, 17 June 2014 (UTC)
- While no disagreement with Antony, to PP I say, forget the IUPAC rigour here: the term has a widely/generally understood meaning. Edgar is correct, but commodity is no less vague, and both are rooted in history of practice. Whether it can be improved or better defined should be the bailiwick of industrial chem experts here, not fly-bys (and not discovery chemists like me, or students or physical chemists). This old chemists opinion. See here for definitions, [14]. Cheers, and hope all is otherwise well, your mate, Le Prof Leprof 7272 (talk) 05:15, 21 June 2014 (UTC)
- I think commodity chemicals is a less-vague synonym for bulk chemicals. Antony–22 (talk⁄contribs) 17:49, 17 June 2014 (UTC)
- Apart from its validity in commerce, where safe transport is the issue, it has a further regulatory niche in storage:
"Regulated Chemical Bulk Storage tanks are defined as (6NYCRR Part 596): (i) an aboveground tank storing a hazardous substance, or mixture thereof, with a capacity of one-hundred and eighty-five (185) gallons or greater; (ii) an underground tank storing a hazardous substance or mixture thereof of any capacity; or (iii) a non-stationary tank used to store one thousand (1,000) kilograms (2,200 lbs.) or more of a hazardous substance or mixture thereof for a period of ninety (90) consecutive days or more." [See [15]]
- You see, after 20-30 years doing something, you can begin to trust instincts. Until then, mate, do the research before acting/suggesting. (You could have found this as well as I.) Le Prof Leprof 7272 (talk) 05:34, 21 June 2014 (UTC)
- Honestly, what are you talking about? I came here for a second opinion, as is appropriate. Plasmic Physics (talk) 06:06, 21 June 2014 (UTC)
- Answered at the appropriate User Talk page. Le Prof Leprof 7272 (talk) 00:14, 22 July 2014 (UTC)
- Honestly, what are you talking about? I came here for a second opinion, as is appropriate. Plasmic Physics (talk) 06:06, 21 June 2014 (UTC)
- Apart from its validity in commerce, where safe transport is the issue, it has a further regulatory niche in storage:
Wikimedia UK and the Royal Society of Chemistry to recruit Wikipedian in Residence - applications welcome
Dear all,
It may be of interest that the Royal Society of Chemistry, an organisation based in Cambridge and London, UK, is looking for a Wikipedian in Residence to deliver a six month full time project. The application closes on 17th August. I hope those of you interested in helping in this project will be encouraged to apply. Details are here.
Many thanks, Daria Cybulska (WMUK) (talk) 15:53, 28 July 2014 (UTC)
Norleucine RM
Hi. Any input at Talk:Norleucine#Requested move would be appreciated. Cheers, Jenks24 (talk) 11:50, 31 July 2014 (UTC)
Dear chemistry experts: Is this old AfC page about a notable topic? Should it be kept instead of being deleted as a stale draft? —Anne Delong (talk) 13:43, 29 July 2014 (UTC)
- Thanks for the notice. I cleaned it up a bit and moved it to article space. -- Ed (Edgar181) 12:30, 31 July 2014 (UTC)
- Great! I see that you made a NAGly redirect, too. —Anne Delong (talk) 03:48, 1 August 2014 (UTC)
Fulminic acid

It seems to me that the right-hand half of this diagram is wrong - the electrons do not add up. It would make since if there ewer a double bond between N and O, a negative charge on the C, a positive charge on the N, and no charge on the O. But it's over 40 years since I studied chemistry, so I'ld like another opinion before I alter it. Maproom (talk) 11:40, 1 August 2014 (UTC)
- I get an electron count of 16 every time: 1+4+4+7, 1+3+5+7. Plasmic Physics (talk) 12:27, 1 August 2014 (UTC)
- I agree. There is an implicit lone-pair on the N in the right diagram (the reaction mechanism for converting left to right is that one of the three pairs shared between C and N pulls back to being solely on N). Seeing the charges is enough explicit detail to determine presence of those non-written electrons. The same analysis is how we know each O actually has 3 implicit lone-pairs (otherwise, each would be +5 not –1). I don't see this mentioned in our skeletal formula, formal charge, or lone pair articles? Yikes! DMacks (talk) 20:38, 1 August 2014 (UTC)
Does anyone else see the issues with this article? It looks like a poorly written essay, with a confused subject. It looks like a random assembly of hydride statements, which after reading, I still don't know what defines a borderline hydride. The alternative definition covers something entirely different, which seems to be only semantically related. Is this article fixable, or should we look at scrapping it? Plasmic Physics (talk) 01:02, 28 July 2014 (UTC)
- The term "borderline hydrides" appears in a handful of books - but mostly as descriptive English, rather than as a defined term. I can find almost no mention of it in journals, so it sounds like OR to me. That said, I like the idea of using dissociative chemisorption as a means of identifying covalent hydrides. We’ve had a lot of difficulty defining the bonding in transition metal hydrides, but presumably any that can form purely by exposure to hydrogen (under normal conditions) must be largely covalent? Project Osprey (talk) 08:53, 28 July 2014 (UTC)
- It is not correct that hydrides formed by simple exposure to hydrogen must be covalent, that forms interstitial hydrides, if anything. How did you get that from the article in any case? Plasmic Physics (talk) 11:44, 28 July 2014 (UTC)
- Shriver and Atkins, a popular inorganic text in the US and UK, uses the term "intermediate hydride" to describe the derivatives of zinc and copper. Greenwood and Earnshaw, which is more scholarly (more detailed, less of a textbook) classify hydrides as "customary to group the binary hydrides ... ionic, metallic, covalent, polymeric, and “intermediate” or “borderline"" but in the next sentence say that this classification "is unsatisfactory because the nature of the bonding is but poorly understood in many cases ... classification obscures the important point that there is an almost continuous gradation in properties - and bond types.." One recommendation might be to convert the article into a sort of disambigution page directing readers to copper hydride and zinc hydride.--Smokefoot (talk) 13:07, 28 July 2014 (UTC)
- I don't know, that does not make a very strong case. That said, I like the idea of converting it into a disambiguation page, although, instead directing to Hydride and Dihydrogen complex. This could be accompanied by merging the contents of Borderline hydrides with those articles. Plasmic Physics (talk) 10:49, 2 August 2014 (UTC)
- Shriver and Atkins, a popular inorganic text in the US and UK, uses the term "intermediate hydride" to describe the derivatives of zinc and copper. Greenwood and Earnshaw, which is more scholarly (more detailed, less of a textbook) classify hydrides as "customary to group the binary hydrides ... ionic, metallic, covalent, polymeric, and “intermediate” or “borderline"" but in the next sentence say that this classification "is unsatisfactory because the nature of the bonding is but poorly understood in many cases ... classification obscures the important point that there is an almost continuous gradation in properties - and bond types.." One recommendation might be to convert the article into a sort of disambigution page directing readers to copper hydride and zinc hydride.--Smokefoot (talk) 13:07, 28 July 2014 (UTC)
- It is not correct that hydrides formed by simple exposure to hydrogen must be covalent, that forms interstitial hydrides, if anything. How did you get that from the article in any case? Plasmic Physics (talk) 11:44, 28 July 2014 (UTC)
C2O4
Here is another possible structure for C2O4:
CO2-O-C=O
The CO2 that is bonded to an oxygen looks like this:
[O=C-O <-> O-C=O]
the O is bonded to 2 carbons.
There is both an oxyanion and a carbocation in this isomer of C2O4.
The negatively charged oxygen reacts with the positively charged carbon and a ring with alternating carbonyls and oxygen atoms forms.
Another possibility is that the O is single bonded to an O and a C which would mean that it is straight.
It looks like this:
O=C-O-O-C=O
there are no oxyanions this time but there are now 2 carbocations instead of 1. This could be stabilized by adding H2 to it or more likely the O-O bond will break and form 2 CO2 molecules and there is even a possibility of the single C-O bonds breaking leaving O2 + 2 CO. — Preceding unsigned comment added by Caters1 (talk • contribs) 13:07, 31 July 2014 (UTC)
- This is not the apppropriate place for this kind of thing. I'm not sure that anywhere on Wikipedia is. I think we should probably delete this section and the preceding one, or put it in a collapsed hat template. 0x0077BE [talk/contrib] 13:11, 31 July 2014 (UTC)
- This I feel is the right place, especially for the one about hydronium being an oxycation. You or some other people could edit the hydronium article to say that it is an oxycation and add both of my structures for C2O4 and information about it including stability and possible bond breakages to the C2O4 article you already have.Caters1 (talk) 14:50, 31 July 2014 (UTC)
- Again, we rely on reliable sources. Nobody is going to add your structures unless they are discussed in a reliable source. When writing here please link the articles you refer to, such as C2O4. --Bduke (Discussion) 21:01, 31 July 2014 (UTC)
- I honestly looked at the molecular formula and looked at possible structural isomers of https://en.wikipedia.org/wiki/C2O4 that you didn't already have on there and I came up with ones that have single C-O bonds that are not between a carbonyl in a ring and an O but rather a C and an O(and for one of them 2 O's) along with the resonance structures for it and even possible intramolecular reactions and bond breakages.Caters1 (talk) 02:09, 1 August 2014 (UTC)
- Let's be very clear. Wikipedia articles (and editors writing them) are absolutely prohibited (WP:SYNTH is the main policy) from including our own hypotheses or personal analysis/extrapolations. There are probably dozens of isomers one could draw for C2O4, especially if one doesn't care about octet rule or other electronic or structural stability details. But unless there are reliable published sources that make the case for them (even if only as a computational analysis for why they would be hard to make), they have no business being listed on the C2O4 page. It's a great mind game (and intro organic-chemistry exam question) try to draw as many "likely to be stable" isomers for a given formula as possible. DMacks (talk) 04:13, 1 August 2014 (UTC)
- Support DMack's comments that one needs "reliable sources". I would add that articles are far stronger if they have a foundation of secondary sources, per WP:SECONDARY. We are mainly looking for topics that have been the subject of a review or discussed in books, usually textbooks. In this way, the article almost automatically meets notability standards and editors avoid synthesis.--Smokefoot (talk) 04:37, 1 August 2014 (UTC)
- For the record, SciFinder lists two additional structural isomers of C2O4 (each having various peroxide bonds in one or more rings) restricting to those that are octet-stable and zero net charge (these also happen to have all zero formal-charges). And then additional ones if net charge ≠0 is allowed. And I can even envision even more (based on known functional groups, just not on this particular substrate) that also are net-zero (and more that are not). But that just proves that reliable sources trump our brainstorming (and demonstrates why only the former is allowed for our encyclopedia). DMacks (talk) 21:48, 1 August 2014 (UTC)
- but my straight one has a peroxide bond. O=C-O-O-C=O. This could then form a 6 membered ring by forming another peroxide bond from the pi bonds(however this is unlikely). You could then add 2Hs to each carbon to make it have an octet and 0 formal charge. You would then have C2H2O4 in the form of a ring with 2 peroxide bonds. This is an isomer of oxalic acid like how the straight chain with 1 peroxide bond is an isomer of C2O4. I can only think of 2 acyclic isomers of C2O4 so that means the rest of them must be 3 membered rings, 4 membered rings, 5 membered rings, and 6 membered rings. You have 2 of the 4 membered ones. 5 membered and above always have at least 1 peroxide bond in the ring whereas 4 membered and below don't always have peroxide bonds.Caters1 (talk) 15:11, 2 August 2014 (UTC)
- Your straight one is original research and by Wikipedia policy is strictly forbidden. Unless you can supply a reliable source that documents its notability, it has absolutely no place in Wikipedia. Full stop. Boghog (talk) 17:22, 2 August 2014 (UTC)
- but my straight one has a peroxide bond. O=C-O-O-C=O. This could then form a 6 membered ring by forming another peroxide bond from the pi bonds(however this is unlikely). You could then add 2Hs to each carbon to make it have an octet and 0 formal charge. You would then have C2H2O4 in the form of a ring with 2 peroxide bonds. This is an isomer of oxalic acid like how the straight chain with 1 peroxide bond is an isomer of C2O4. I can only think of 2 acyclic isomers of C2O4 so that means the rest of them must be 3 membered rings, 4 membered rings, 5 membered rings, and 6 membered rings. You have 2 of the 4 membered ones. 5 membered and above always have at least 1 peroxide bond in the ring whereas 4 membered and below don't always have peroxide bonds.Caters1 (talk) 15:11, 2 August 2014 (UTC)
- Let's be very clear. Wikipedia articles (and editors writing them) are absolutely prohibited (WP:SYNTH is the main policy) from including our own hypotheses or personal analysis/extrapolations. There are probably dozens of isomers one could draw for C2O4, especially if one doesn't care about octet rule or other electronic or structural stability details. But unless there are reliable published sources that make the case for them (even if only as a computational analysis for why they would be hard to make), they have no business being listed on the C2O4 page. It's a great mind game (and intro organic-chemistry exam question) try to draw as many "likely to be stable" isomers for a given formula as possible. DMacks (talk) 04:13, 1 August 2014 (UTC)
- I honestly looked at the molecular formula and looked at possible structural isomers of https://en.wikipedia.org/wiki/C2O4 that you didn't already have on there and I came up with ones that have single C-O bonds that are not between a carbonyl in a ring and an O but rather a C and an O(and for one of them 2 O's) along with the resonance structures for it and even possible intramolecular reactions and bond breakages.Caters1 (talk) 02:09, 1 August 2014 (UTC)
- Again, we rely on reliable sources. Nobody is going to add your structures unless they are discussed in a reliable source. When writing here please link the articles you refer to, such as C2O4. --Bduke (Discussion) 21:01, 31 July 2014 (UTC)
- This I feel is the right place, especially for the one about hydronium being an oxycation. You or some other people could edit the hydronium article to say that it is an oxycation and add both of my structures for C2O4 and information about it including stability and possible bond breakages to the C2O4 article you already have.Caters1 (talk) 14:50, 31 July 2014 (UTC)
The uses of Particulate, Particulates, Particulate matter is under discussion, see talk:Particulates -- 65.94.169.222 (talk) 04:35, 4 August 2014 (UTC)
