Tosylhydrazone
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.[1]
Synthesis
[edit]As an example camphor tosylhydrazone is synthesised from camphor and tosylhydrazine in ethanol with hydrochloric acid catalysis.[2]
Reactions
[edit]Hydrolysis is the reverse reaction of formation with regeneration of the carbonyl compound.
In the Shapiro reaction tosylhydrazones are used as a leaving group in elimination reactions. This reaction requires a strong base. If sodium methoxide is used as the base the reaction is called a Bamford–Stevens reaction. Tosylhydrazones can be reduced to the corresponding alkanes with reagents such as sodium borohydride and borane.
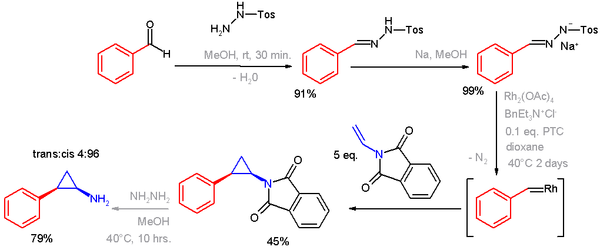
Tosylhydrazone salts can react with metals to form metal carbenes and used in cyclopropanations and epoxidations.[3][4] An example of a transition metal-catalyzed cyclopropanation is a synthesis of tranylcypromine,[5][6] in which the sodium salt of benzaldehyde tosylhydrazone is converted to a rhodium metal carbene through the diazo intermediate.
Tosylhydrazones are also starting materials for certain cross-coupling reactions.[7] In the first report on this reaction type the coupling partners were a tosylhydrazone, an aryl halide with catalyst system dibenzylideneacetone / XPhos.[8] As part of the catalytic cycle the diazo intermediateformed by decomposition of the tosylhydrazone forms a palladium-carbene complex with the oxidative addition complex of palladium with the aryl halide. Using this powerful method it is possible to access bioactive compounds.[9] [10]
References
[edit]- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- ^ 2-Bornene, Organic Syntheses, Coll. Vol. 6, p.172 (1988); Vol. 51, p.66 (1971). link
- ^ A New Protocol for the In Situ Generation of Aromatic, Heteroaromatic, and Unsaturated Diazo Compounds and Its Application in Catalytic and Asymmetric Epoxidation of Carbonyl Compounds. Extensive Studies To Map Out Scope and Limitations, and Rationalization of Diastereo- and Enantioselectivities Varinder K. Aggarwal, Emma Alonso, Imhyuck Bae, George Hynd, Kevin M. Lydon, Matthew J. Palmer, Mamta Patel,, Marina Porcelloni, Jeffery Richardson, Rachel A. Stenson, John R. Studley, Jean-Luc Vasse, and Caroline L. Winn Journal of the American Chemical Society 2003 125 (36), 10926-10940 doi:10.1021/ja034606+
- ^ Fulton, J. R., Aggarwal, V. K. and de Vicente, J. (2005), The Use of Tosylhydrazone Salts as a Safe Alternative for Handling Diazo Compounds and Their Applications in Organic Synthesis. European Journal of Organic Chemistry, 2005: 1479–1492. doi:10.1002/ejoc.200400700
- ^ Catalytic Cyclopropanation of Alkenes Using Diazo Compounds Generated in Situ. A Novel Route to 2-Arylcyclopropylamines Varinder K. Aggarwal, Javier de Vicente, and Roger V. Bonnert Org. Lett.; 2001; 3(17) pp 2785 - 2788; (Letter) doi:10.1021/ol0164177
- ^ Notes: benzaldehyde reacts with p-toluenesulfonyl hydrazide to the hydrazone.Its sodium salt reacts with N-Vinylphthalimide and rhodium acetate, a phase transfer catalyst and PTC to the cyclopropane. The phthalimide group is removed by hydrazine. The product is the cis isomer but by switching to ClFeTPP the amount of trans isomer increases to 33%
- ^ Barluenga, J. and Valdés, C. (2011), Tosylhydrazones: New Uses for Classic Reagents in Palladium-Catalyzed Cross-Coupling and Metal-Free Reactions. Angewandte Chemie International Edition, 50: 7486–7500. doi:10.1002/anie.201007961
- ^ Barluenga, J., Moriel, P., Valdés, C. and Aznar, F. (2007), N-Tosylhydrazones as Reagents for Cross-Coupling Reactions: A Route to Polysubstituted Olefins. Angewandte Chemie International Edition, 46: 5587–5590. doi:10.1002/anie.200701815
- ^ E. Brachet, A. Hamze, J.-F. Peyrat, J.-D. Brion, M. Alami, Org. Lett., 2010, 12 (18), pp 4042–4045 doi:10.1021/ol101639g
- ^ Aziz, J.; Brachet, E.; Hamze, A.; Peyrat, J.-F.; Bernadat, G.; Morvan, E.; Bignon, J.; Wdzieczak-Bakala, J.; Dubois, J.; Tueni, M.; Yassine, A.; Brion, J.-D.; Alami, M.; Synthesis, biological evaluation, and structure-activity relationships of tri- and tetrasubstituted olefins related to isoCombretastatin A-4 as new tubulin inhibitors. Org. Biomol. Chem., 2013,11, 430-442. doi:10.1039/C2OB26253C