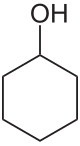
Cyclohexanol
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexanol | |||
| Other names
Cyclohexyl Alcohol,
hexahydrophenol, hydrophenol, hydroxycyclohexane, Naxol Hexalin Hydralin HOCy | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.301 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O | |||
| Molar mass | 100.158 g/mol | ||
| Appearance | Colorless, viscous liquid. Hygroscopic | ||
| Odor | camphor-like | ||
| Density | 0.9624 g/mL, liquid | ||
| Melting point | 25.93 °C (78.67 °F; 299.08 K) | ||
| Boiling point | 161.84 °C (323.31 °F; 434.99 K) | ||
| 3.60 g/100 mL (20 °C) 4.3 g/100 mL (30 °C) | |||
| Solubility | soluble in ethanol, ethyl ether, acetone, chloroform miscible with ethyl acetate, linseed oil, benzene | ||
| Vapor pressure | 1 mmHg (20°C)[2] | ||
Henry's law
constant (kH) |
4.40 x 10−6 atm-cu m/mol | ||
| Acidity (pKa) | 16 | ||
| -73.40·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4641 | ||
| Viscosity | 41.07 mPa·s (30 °C) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, skin irritant Reacts violently with oxidizing agents | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H302, H315, H332, H335 | |||
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P321, P330, P332+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 67 °C (153 °F; 340 K) | ||
| 300 °C (572 °F; 573 K) | |||
| Explosive limits | 2.7-12% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2060 mg/kg (oral, rat) 2200-2600 mg/kg (oral, rabbit)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 50 ppm (200 mg/m3)[2] | ||
REL (Recommended)
|
TWA 50 ppm (200 mg/m3)[2] | ||
IDLH (Immediate danger)
|
400 ppm[2] | ||
| Safety data sheet (SDS) | MSDS for cyclohexanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclohexanol is the organic compound with the formula HOCH(CH2)5. The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group.[4] This compound exists as a deliquescent colorless solid with a camphor-like odor, which, when very pure, melts near room temperature. Millions of tonnes are produced annually, mainly as a precursor to nylon.[5]
Production
[edit]Cyclohexanol is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts:[5]
- 2 C6H12 + O2 → 2 C6H11OH
This process coforms cyclohexanone, and this mixture ("KA oil" for ketone-alcohol oil) is the main feedstock for the production of adipic acid. The oxidation involves radicals and the intermediacy of the hydroperoxide C6H11O2H. Alternatively, cyclohexanol can be produced by the hydrogenation of phenol:
- C6H5OH + 3 H2 → C6H11OH
This process can also be adjusted to favor the formation of cyclohexanone.
Basic reactions
[edit]Cyclohexanol undergoes the main reactions expected for a secondary alcohol. Oxidation gives cyclohexanone, which is converted on a large scale in industry to the oxime, a precursor to caprolactam. As a laboratory exercise, this oxidation can be effected with chromic acid. Esterification affords the commercially useful derivatives dicyclohexyladipate and dicyclohexylphthalate, which are used as plasticizers. Heating in the presence of acid catalysts converts cyclohexanol to cyclohexene.[5][6]
Structure
[edit]Cyclohexanol has at least two solid phases. One of them is a plastic crystal.
Applications
[edit]As indicated above, cyclohexanol is an important feedstock in the polymer industry, firstly as a precursor to nylons, but also to various plasticizers. Small amounts are used as a solvent.
Safety
[edit]Cyclohexanol is moderately toxic: the Threshold Limit Value for the vapor for 8 h is 50 ppm.[5] The IDLH concentration is set at 400 ppm, based on studies on the acute oral toxicity in animals.[7] Few studies have been done on its carcinogenicity, but one study on rats found it to have co-carcinogenic effects.[8]
References
[edit]- ^ Merck Index, 11th Edition, 2731.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0165". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Cyclohexanol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- ^ a b c d Michael Tuttle Musser "Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- ^ G. H. Coleman, H. F. Johnstone (1925). "Cyclohexene". Organic Syntheses. 5: 33. doi:10.15227/orgsyn.005.0033.
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards
- ^ [1] Lucrecia Márquez-Rosado, Cristina Trejo-Solís 2, María del Pilar Cabrales-Romero, Evelia Arce-Popoca, Adolfo Sierra-Santoyo, Leticia Alemán-Lazarini, Samia Fatel-Fazenda, Claudia E. Carrasco-Legleu, Saúl Villa-Treviño, "Co-carcinogenic effect of cyclohexanol on the development of preneoplastic lesions in a rat hepatocarcinogenesis model", Molecular Carcinogenesis, Vol. 46, No. 7, Pages 524 - 533, March 2007.