ZNF837
| ZNF837 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ZNF837, zinc finger protein 837 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | HomoloGene: 138182; GeneCards: ZNF837; OMA:ZNF837 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
ZNF837 is a protein that in humans is encoded by the ZNF837 gene,[3] is located at 19q13.431 with minus strand orientation.[4] ZNF837 protein is characterized as a C2H2-type zinc finger protein.[5]
Homology and Evolution
[edit]
The human ZNF837 has homologs present in many mammals and seen more distantly. All homologs are chordates. All contain both COG5048 and Zf-C2H2_2 domains. The areas that these domains are found contain the highest conservation rates. In humans, 5 Zf-H2C2 double domains[6] and 2 COG5048 domains[7] are present.
The protein sequence is fast evolving among these homologs.
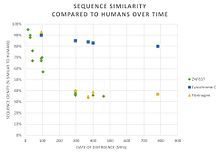
ZNF837 has numerous paralogs in humans, all of which are zinc finger proteins.
Human ZNF837
[edit]In humans, there are no other aliases, and its neighboring genes are MIR4754, A1BG, and RPS5. ZNF837 mRNA that is made into function protein contains 1921 nucleotides, of which 222-1817 are translated to a protein containing 3 exons. The protein consists of 531 amino acids[8] with a weight of 58,078 Da with an isoelectric point at 9.525.[9]
Gene Variants
[edit]There are 2 transcript variants. Transcript variant 1, 2050 base pairs in length, is non-coding due to a nonsense-mediated mRNA decay .[10] Transcript variant 2 is made into the functional protein due to an alternate splice site[11]
Post Translational
[edit]It is predicted via high conservation to have 4 phosphorylation sites[12] at T386, T455, S460, Y503.[13] The internal structure is includes combination of alpha helices, beta sheets and mainly random coils.[14]

Expression
[edit]ZNF837 has observed in the pancreas, liver, uterus, and muscle cells. In all cases concentration is low.[15] However, the expression of ZNF837 is seen to have the most impact is when looking at normal vs diseased state. There is a consistent change that is able to be seen.
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000152475 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Strausberg, RL; Feingold, EA; Grouse, LH; et al. (December 2002). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proceedings of the National Academy of Sciences. 99 (26): 16899–16903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- ^ "ZNF837 zinc finger protein 837 [Homo sapiens (human)] - Gene - NCBI".
- ^ Pieler, T.; Bellefroid, E. (1994). "Perspectives on zinc finger protein function and evolution - an update". Molecular Biology Reports. 20 (1): 1–8. doi:10.1007/bf00999848. PMID 7531280. S2CID 22962225.
- ^ Iuchi, S (2001). "Three classes of C2H2 zinc finger proteins". Cellular and Molecular Life Sciences. 58 (4): 625–635. doi:10.1007/pl00000885. PMC 11146492. PMID 11361095. S2CID 6522993.
- ^ "NCBI Conserved Domain Search".
- ^ "ZNF837 protein [Homo sapiens] - Protein - NCBI".
- ^ "ZNF837 (human)".
- ^ Maquat, Lynne E (2002). "Nonsense-mediated mRNA decay". Current Biology. 12 (6): R196 – R197. doi:10.1016/S0960-9822(02)00747-9. PMID 11909543.
- ^ "ZNF837 (human)".
- ^ Blom, Nikolaj; Gammeltoft, Steen; Brunak, Søren (1999). "Sequence and structure-based prediction of eukaryotic protein phosphorylation sites 1". Journal of Molecular Biology. 294 (5): 1351–1362. doi:10.1006/jmbi.1999.3310. PMID 10600390.
- ^ Guo, Ailan et al. Serine, Threonine, and Tyrosine Phosphorylation Sites. Patent US20110045603 A1. 20 Apr. 2010. Print.
- ^ Kelley, LA; Sternberg, MJE (2009). "Protein structure prediction on the Web: a case study using the Phyre server". Nature Protocols. 4 (3): 363–371. doi:10.1038/nprot.2009.2. hdl:10044/1/18157. PMID 19247286. S2CID 12497300.
- ^ "EST Profile - Hs.222236".


