Wikipedia talk:WikiProject Chemicals/Archive 2023
| This is an archive of past discussions on Wikipedia:WikiProject Chemicals. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 2020 | Archive 2021 | Archive 2022 | Archive 2023 | Archive 2024 |
Re: TOXNET has moved
Reviving this old thread. I just came across a broken ref link using {{HPD}}, so obviously nothing has been done about this yet and it still needs doing. Since it's only used on 30-some-odd pages, replacing them all with a hardcoded link to the appropriate CPID page wouldn't be too hard, but... if anyone can think of a way to modify just the template to fix all the links, that would be preferable -- if it's even possible, and it may not be. I haven't found the old chemical ID numbers anywhere on the new site yet. Thoughts?
-Garrett W. {☎ ✍} 20:13, 29 December 2022 (UTC)
- The template might be amended as in the de-WP version (diff). --Leyo 21:51, 1 January 2023 (UTC)
Toad toxins move
If this move (reasoned with https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/1469860166.pdf) was correct, what about Category:Bufanolides and Bufanolide? --Leyo 08:42, 4 January 2023 (UTC)
- @Leyo The quoted article says
Bufadienolides or compounds with similar structures are not only found in toads but also in many plant species, fireflies (Photinus sp.), Snakes (Rhabdophis sp.) and mammals (Steyn[57] and Heerden 1998)
. Thus it is arguably a mistake to place these compounds only within a "toad toxin" category. Our article section at Bufadienolide#Classification gives the full MeSH classification. Mike Turnbull (talk) 12:12, 4 January 2023 (UTC)- Thank you for the feedback. @Yyfroy and Túrelio: Pinging the two involved users on Commons. --Leyo 15:05, 4 January 2023 (UTC)
- Bufadienolides and bufanolides are two distinct categories, but MeSH puts them in the same category! That's not logical!And I'm wondering why "https://en.wikipedia.org/wiki/Category:Bufanolides" still exists! It is outrageous that "bufadienolide belongs to Category:Bufanolides"!
- Choosing "Toad toxins" as the catergory's name may not be perfect, but it's like humans can have swine flu.Yyfroy (talk) 04:09, 5 January 2023 (UTC)
- If there are actual structure classes of chemicals, each should have its own category with a specific name. If one class is a subclass of another, then that's easily accomodated too. But if some of the chemicals happen to come from a certain source or have a certain lay-language description, that can be a distinct type of categorization. DMacks (talk) 04:22, 5 January 2023 (UTC)
Various chemicals articles that attract much attention
Some chemicals that attract so much attention:
- Dimanganese heptoxide is one, a volatile derivative of permanganate. About 120 views/day. Totally useless.
- Hexafluoroantimonic acid, a superacid. About 500 views/day. There is so much interest in its strength, sort of a testosterone thing: "my acid is stronger than yours".
- Thioacetone, which is misrepresented because it unlikely to exist as shown. Its really smelly but so what? 1200 views/day.
- Xenon hexafluoroplatinate. Its not even a compound. 43 views/day.
--Smokefoot (talk) 20:14, 12 January 2023 (UTC)
Oh, I like this game!
- Arsole, putrid smell, puerile humour. 27 views a day. (sorry...)
Anyway... I was curious so I did some digging. Fluoroantimonic acid has 279 results on google scholar but 22,700 results in youtube. Manganese heptoxide is also very popular (instant fire!). I'm not sure what's driving the other two, maybe TikTok? --Project Osprey (talk) 23:19, 12 January 2023 (UTC)
- I'm not sure if it's a good thing or a bad thing we don't have arsepane. DMacks (talk) 15:47, 13 January 2023 (UTC)
- I'm not finding much in the literature about arsepane. It's kind of irritating, but I guess it will have to stay red for a while. DMacks (talk) 15:49, 13 January 2023 (UTC)
- Probably for the best, it's a ugly looking ring. --Project Osprey (talk) 16:16, 13 January 2023 (UTC)
- I'm not finding much in the literature about arsepane. It's kind of irritating, but I guess it will have to stay red for a while. DMacks (talk) 15:49, 13 January 2023 (UTC)
- The xenon-containing species in xenon hexafluoroplatinate was the first noble gas compound to be discovered. –LaundryPizza03 (dc̄) 15:48, 13 January 2023 (UTC)
- Sorta, but there is no compound there.--Smokefoot (talk) 17:39, 13 January 2023 (UTC)
Chembox: adding AITS spectral database external link (SDBS)
- See Wikipedia talk:WikiProject Chemistry § Linking Chembox to spectroscopic data on the AITS database (AITS: SDBS database external link).
DePiep (talk) 11:46, 19 January 2023 (UTC)
Unreviewed Featured articles year-end summary
Unreviewed featured articles/2020/4Q2022
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Restoring older Featured articles to standard:year-end 2022 summary
Unreviewed featured articles/2020 (URFA/2020) is a systematic approach to reviewing older Featured articles (FAs) to ensure they still meet the FA standards. A January 2022 Signpost article called "Forgotten Featured" explored the effort. Progress is recorded at the monthly stats page. Through 2022, with 4,526 very old (from the 2004–2009 period) and old (2010–2015) FAs initially needing review:
Of the FAs kept, deemed satisfactory by three reviewers, or delisted, about 60% had prior review between 2004 and 2007; another 20% dated to the period from 2008–2009; and another 20% to 2010–2015. Roughly two-thirds of the old FAs reviewed have retained FA status or been marked "satisfactory", while two-thirds of the very old FAs have been defeatured. Entering its third year, URFA is working to help maintain FA standards; FAs are being restored not only via FAR, but also via improvements initiated after articles are reviewed and talk pages are noticed. Since the Featured Article Save Award (FASA) was added to the FAR process a year ago, 38 FAs were restored to FA status by editors other than the original FAC nominator. Ten FAs restored to status have been listed at WP:MILLION, recognizing articles with annual readership over a million pageviews, and many have been rerun as Today's featured article, helping increase mainpage diversity.
But there remain almost 4,000 old and very old FAs to be reviewed. Some topic areas and WikiProjects have been more proactive than others in restoring or maintaining their old FAs. As seen in the chart below, the following have very high ratios of FAs kept to those delisted (ordered from highest ratio):
and others have a good ratio of kept to delisted FAs:
... so kudos to those editors who pitched in to help maintain older FAs !
But looking only at the oldest FAs (from the 2004–2007 period), there are 12 content areas with more than 20 FAs still needing review: Biology, Music, Royalty and nobility, Media, Sport and recreation, History, Warfare, Meteorology, Physics and astronomy, Literature and theatre, Video gaming, and Geography and places. In the coming weeks, URFA/2020 editors will be posting lists to individual WikiProjects with the goal of getting these oldest-of-the-old FAs reviewed during 2023. Ideas for how you can help are listed below and at the Signpost article.
More regular URFA and FAR reviewers will help assure that FAs continue to represent examples of Wikipedia's best work. If you have any questions or feedback, please visit Wikipedia talk:Unreviewed featured articles/2020/4Q2022. |
FAs last reviewed from 2004 to 2007 of interest to this WikiProject
If you review an article on this list, please add commentary at the article talk page, with a section heading == [[URFA/2020]] review== and also add either Notes or Noticed to WP:URFA/2020A, per the instructions at WP:URFA/2020. Comments added here may be swept up in archives and lost, and more editors will see comments on article talk. SandyGeorgia (Talk) 21:11, 20 January 2023 (UTC)
- Enzyme kinetics
- Francium
- Joseph Priestley
- Nicotinamide adenine dinucleotide
- Oxidative phosphorylation
- Uranium
- Xenon
Should certain organic compounds have a "Variants" section?
Inorganic compounds like sodium chloride often have a short "Related compounds" section in their infobox, because there are only so many related binary salts you can make. Lithium chloride, potassium chloride, sodium fluoride, sodium bromide, etc. This isn't practical for organic compounds though, because there's hundreds to thousands of different variations of an organic compound, just from different functional groups you can add on, isomers. Should "base" organic compounds have a variants section, containing variants which have articles, which can be moved into a separate article if it gets long enough? For examples I just made, see Phenylacetic acid, Dibenzyl ketone, and Tetraphenylcyclopentadienone. This would help make articles easier to find by weaving the web of links. Then hypothetically long lists could be moved to e.g. "List of phenylacetic acid variants". Michael7604 (talk) 21:03, 20 January 2023 (UTC)
- @Michael7604 I don't like that idea: as well as the reason you mention, "variants" are in the eye of the beholder. To take a specific example, acetone does have a "related compounds" section which currently has Butanone, Isopropyl alcohol, Formaldehyde, Urea and Carbonic acid. To my eye, the relationship between acetone and urea is tenuous at best and downright misleading at worst. Professional organic chemists use the word analog in a way that is still not very precise but usually refers to some sort of Markush structure, especially in relation to patents. Mike Turnbull (talk) 22:32, 20 January 2023 (UTC)
- One idea, maybe the criteria being counted as a "related compound" could be if the name contains the main compound. E.g., 4-bromophenyl acetic acid contains "phenylacetic acid". One of the names of butanone, "methylacetone", contains "Acetone". But isopropyl alcohol has nothing to do with "acetone". This would also apply if the name gets split up, e.g. Bis(4-bromobenzyl) ketone is a variant of Dibenzyl ketone. With this criteria just adding a functional group to a molecule always makes it a related compound. One way or another there should be a way for curious readers to learn about similar compounds, since mobile readers can't see categories. Michael7604 (talk) 22:40, 20 January 2023 (UTC)
- That would be a good argument for getting the mediawiki folk to add such a facility. Note that we do have a system of templates that is much superior, in my opinion. Hence for a herbicide like 2,4-D, the only "related compound" in the chembox is 2,4,5-T but at the bottom of the article there's a template {{herbicides}} that does a very good job of linking to relevant additional articles. Mike Turnbull (talk) 22:56, 20 January 2023 (UTC)
- @Michael D. Turnbull Footer templates aren't visible for mobile either, that is why it is important to weave the web with links in article bodies. Michael7604 (talk) 23:05, 20 January 2023 (UTC)
- First, proposals like this are healthy, so thank you Michael7604. Second, however, I'm 95% with Michael D. Turnbull on being opposed. One reason is that selection of variants is just too subjective. And the idea invites contributions from editors who know isolated factoids but lack an appreciation of the landscape of organic chemistry. Then the handful of knowledgeable editors would be forced into doing a lot more pruning vs content creation, our prime mission. On the other hand, there is nothing to stop an editor from adding an ad hoc "related compound" as someone did today on 2-octanone by noting its relevance to filbertone. Filbertone is not really a variant, but the link nicely complements our article, IMHO.--Smokefoot (talk) 23:56, 20 January 2023 (UTC)
- @Michael D. Turnbull Footer templates aren't visible for mobile either, that is why it is important to weave the web with links in article bodies. Michael7604 (talk) 23:05, 20 January 2023 (UTC)
- That would be a good argument for getting the mediawiki folk to add such a facility. Note that we do have a system of templates that is much superior, in my opinion. Hence for a herbicide like 2,4-D, the only "related compound" in the chembox is 2,4,5-T but at the bottom of the article there's a template {{herbicides}} that does a very good job of linking to relevant additional articles. Mike Turnbull (talk) 22:56, 20 January 2023 (UTC)
- One idea, maybe the criteria being counted as a "related compound" could be if the name contains the main compound. E.g., 4-bromophenyl acetic acid contains "phenylacetic acid". One of the names of butanone, "methylacetone", contains "Acetone". But isopropyl alcohol has nothing to do with "acetone". This would also apply if the name gets split up, e.g. Bis(4-bromobenzyl) ketone is a variant of Dibenzyl ketone. With this criteria just adding a functional group to a molecule always makes it a related compound. One way or another there should be a way for curious readers to learn about similar compounds, since mobile readers can't see categories. Michael7604 (talk) 22:40, 20 January 2023 (UTC)
This "variant" problem is worse in simple aromatic rings. Some articles like oxazole basically hard to separate the parent compound and everything that have this ring. --Nucleus hydro elemon (talk) 09:37, 21 January 2023 (UTC)
Articles with long titles
When the title of an article is too long and it doesn't contain any hyphens enabling a line break, there might be a layout issue: The chembox is then moved to the left (see e.g. Methoxymethylenetriphenylphosphorane). These articles might be affected: hastemplate:chembox intitle:/[A-Z\(\)]{33}/i (redirects filtered out) --Leyo 08:09, 20 January 2023 (UTC)
- I cannot reproduce this on my browser, Google Chrome in macOS Monterey. –LaundryPizza03 (dc̄) 08:23, 20 January 2023 (UTC)
- At the even longer title Phosphoribosylaminoimidazolesuccinocarboxamide (46 letters), it just made the chembox wider than, say, Pyran (5 letters). –LaundryPizza03 (dc̄) 08:28, 20 January 2023 (UTC)
- I use Firefox. In Edge, it is the same as you describe for Google Chrome. Anyway, both widening the chembox and moving it leftwards is undesirable. --Leyo 10:52, 20 January 2023 (UTC)
- (ec) How should the chembox handle this? Hard to automate, better require an editor to add {{SHY}}. We could start with categorising when title length > n. DePiep (talk) 11:24, 20 January 2023 (UTC)
- Applied {{SHY}} to
|Name=. See Methoxymethylenetriphenylphosphorane [1] (at the right place?) DePiep (talk) 11:26, 20 January 2023 (UTC)- @Leyo @LaundryPizza03 @DePiep The problem is easily solved by using the |Name parameter to give the image a shorter name and override the default. I've just done that for Phosphoribosylaminoimidazolesuccinocarboxamide, so you can see the result. Mike Turnbull (talk) 11:26, 20 January 2023 (UTC)
- See also 5′-Phosphoribosyl-4-carboxy-5-aminoimidazole: these automated breaks are OK right away? -DePiep (talk) 11:30, 20 January 2023 (UTC)
- And DePiep's alternative using {{SHY}} is also fine if you instead want to add a hyphen. Mike Turnbull (talk) 11:30, 20 January 2023 (UTC)
- @DePiep and Michael D. Turnbull: Thanks. Have you gone through the whole list? Or have you got a search link with less false positives? --Leyo 22:51, 22 January 2023 (UTC)
- re Leyo. I changed the regex (add
\detc), found ca. 850 article titles of which 600 are redirects. Resulting List is at User:DePiep/sandbox2. Also in therekept some ~50 really long Redirect names (because: in target article, is the infobox title shortened for this wrong reason?). Redlinks must be typo's from my cleanup. (Source cannot output raw datalist). - It's yours to use (c/p), I don't have time to check them. DePiep (talk) 07:24, 23 January 2023 (UTC)
- Thank you. There seem to be quite some with hyphens (i.e. an optional line break). --Leyo 10:41, 23 January 2023 (UTC)
- @Leyo, @DePiep I've extracted DePiep's list into an Excel spreadsheet and easily found the names where there is already a hyphen or a blank that allows the names to line-break automatically and hence no need for the {{shy}} trick. I'll go through all 86 articles that I have found which do need a soft hyphen and put one in. I should easily complete this today. Mike Turnbull (talk) 11:38, 23 January 2023 (UTC)
- Yes, formal in-name true character U+002D - HYPHEN-MINUS will do some linebreaking already. But it is my impression that these (chemistry name induced) hyphens might be positioned bad for linebreaking. Again, {{SHY}} is our friend. DePiep (talk) 11:39, 23 January 2023 (UTC)
- OK all done, I think. Any left over can be done as people spot them. Mike Turnbull (talk) 12:32, 23 January 2023 (UTC)
- Thank you. I found 18 additional articles that I changed accordingly. --Leyo 16:43, 23 January 2023 (UTC)
- OK all done, I think. Any left over can be done as people spot them. Mike Turnbull (talk) 12:32, 23 January 2023 (UTC)
- Thank you. There seem to be quite some with hyphens (i.e. an optional line break). --Leyo 10:41, 23 January 2023 (UTC)
- re Leyo. I changed the regex (add
- @DePiep and Michael D. Turnbull: Thanks. Have you gone through the whole list? Or have you got a search link with less false positives? --Leyo 22:51, 22 January 2023 (UTC)
- And DePiep's alternative using {{SHY}} is also fine if you instead want to add a hyphen. Mike Turnbull (talk) 11:30, 20 January 2023 (UTC)
- See also 5′-Phosphoribosyl-4-carboxy-5-aminoimidazole: these automated breaks are OK right away? -DePiep (talk) 11:30, 20 January 2023 (UTC)
- @Leyo @LaundryPizza03 @DePiep The problem is easily solved by using the |Name parameter to give the image a shorter name and override the default. I've just done that for Phosphoribosylaminoimidazolesuccinocarboxamide, so you can see the result. Mike Turnbull (talk) 11:26, 20 January 2023 (UTC)
- Applied {{SHY}} to
- (ec) How should the chembox handle this? Hard to automate, better require an editor to add {{SHY}}. We could start with categorising when title length > n. DePiep (talk) 11:24, 20 January 2023 (UTC)
- I use Firefox. In Edge, it is the same as you describe for Google Chrome. Anyway, both widening the chembox and moving it leftwards is undesirable. --Leyo 10:52, 20 January 2023 (UTC)
- Long ago when chembox validation was getting started, we centralized on not inserting anything that would make the display appear different than the actual name, in order to improve discoverability from external search-engines. DMacks (talk) 10:49, 23 January 2023 (UTC)
- 1. By adding {{SHY}} in
|Name=, the article title is not changed. - 2. The external search argument is handled by {{DISPLAYTITLE}}. {{Chembox}} is not to make exceptions in this. DePiep (talk) 11:31, 23 January 2023 (UTC)
- 1. By adding {{SHY}} in
Psilocybin Featured article review
User:DigitalIceAge has nominated Psilocybin for a featured article review here. Please join the discussion on whether this article meets the featured article criteria. Articles are typically reviewed for two weeks. If substantial concerns are not addressed during the review period, the article will be moved to the Featured Article Removal Candidates list for a further period, where editors may declare "Keep" or "Delist" in regards to the article's featured status. The instructions for the review process are here. SandyGeorgia (Talk) 11:25, 24 January 2023 (UTC)
Good article reassessment for Iron(III) chloride
Iron(III) chloride has been nominated for a good article reassessment. If you are interested in the discussion, please participate by adding your comments to the reassessment page. If concerns are not addressed during the review period, the good article status may be removed from the article. Nucleus hydro elemon (talk) 08:16, 27 January 2023 (UTC)
Discussion at WP:MCQ § Proactive request for input
![]() You are invited to join the discussion at WP:MCQ § Proactive request for input. -- Marchjuly (talk) 19:42, 23 February 2023 (UTC)
You are invited to join the discussion at WP:MCQ § Proactive request for input. -- Marchjuly (talk) 19:42, 23 February 2023 (UTC)
Project-independent quality assessments
Quality assessments are used by Wikipedia editors to rate the quality of articles in terms of completeness, organization, prose quality, sourcing, etc. Most wikiprojects follow the general guidelines at Wikipedia:Content assessment, but some have specialized assessment guidelines. A recent Village pump proposal was approved and has been implemented to add a |class= parameter to {{WikiProject banner shell}}, which can display a general quality assessment for an article, and to let project banner templates "inherit" this assessment.
No action is required if your wikiproject follows the standard assessment approach. Over time, quality assessments will be migrated up to {{WikiProject banner shell}}, and your project banner will automatically "inherit" any changes to the general assessments for the purpose of assigning categories.
However, if your project has decided to "opt out" and follow a non-standard quality assessment approach, all you have to do is modify your wikiproject banner template to pass {{WPBannerMeta}} a new |QUALITY_CRITERIA=custom parameter. If this is done, changes to the general quality assessment will be ignored, and your project-level assessment will be displayed and used to create categories, as at present. Aymatth2 (talk) 13:19, 10 April 2023 (UTC)
Stylized depiction of chemical structures
IMHO the dot structures found e.g. in Croconate blue, Croconate violet, Oxocarbon, Peroxydicarbonate, 1,3-Bis(dicyanomethylene)squarate and 1,2-Bis(dicyanomethylene)squarate do not comply with MOS:CHEM. Leyo 15:09, 18 April 2023 (UTC)
- Agreed. They seem to have been added by Jorge Stolfi. The one I checked was done in 2010. Jorge seems to still be active (last edit this March) so perhaps he would like to change the drawings to more conventional ones, or I can do that if he is no longer interested. Mike Turnbull (talk) 16:23, 18 April 2023 (UTC)
- I have now redrawn the individual compounds Croconate blue, Croconate violet, 1,3-Bis(dicyanomethylene)squarate and 1,2-Bis(dicyanomethylene)squarate. Another I've changed is 2-(Dicyanomethylene)croconate. The oxocarbon article has lots of drawing that are consistent, although not aligned with usual conventions, and I've left that alone, along with peroxydicarbonate. Mike Turnbull (talk) 15:59, 21 April 2023 (UTC)
- Though perhaps the different image version should have a different name. Graeme Bartlett (talk) 12:10, 22 April 2023 (UTC)
- @Graeme Bartlett I would have done that if any of the articles in which the diagrams were used had a reason to retain the old images but I could see no justification for that in MOS:CHEM. Of course, if the earlier versions were needed, they can easily be obtained from the Commons history and I would have no objections if my overwrites were reverted in that case. Mike Turnbull (talk) 17:19, 22 April 2023 (UTC)
- That's not allowed by commons guidelines (commons:COM:OVERWRITE). Images that are substantively different need to be uploaded at a different filename. Please undo/revert those changes there. DMacks (talk) 18:15, 22 April 2023 (UTC)
- c:COM:SPLIT exists to handle those cases while preserving history and avoiding unnecessary complications. –LaundryPizza03 (dc̄) 18:39, 22 April 2023 (UTC)
- Thanks for the link. I've put in the splitting requests. Mike Turnbull (talk) 20:49, 22 April 2023 (UTC)
- For the record, in order of amount of total work required: someone else handling your split-request > you re-uploading at a different name and reverting the old filename's contents > you filing a split-request. DMacks (talk) 03:42, 23 April 2023 (UTC)
- Thanks for the link. I've put in the splitting requests. Mike Turnbull (talk) 20:49, 22 April 2023 (UTC)
- c:COM:SPLIT exists to handle those cases while preserving history and avoiding unnecessary complications. –LaundryPizza03 (dc̄) 18:39, 22 April 2023 (UTC)
- That's not allowed by commons guidelines (commons:COM:OVERWRITE). Images that are substantively different need to be uploaded at a different filename. Please undo/revert those changes there. DMacks (talk) 18:15, 22 April 2023 (UTC)
- @Graeme Bartlett I would have done that if any of the articles in which the diagrams were used had a reason to retain the old images but I could see no justification for that in MOS:CHEM. Of course, if the earlier versions were needed, they can easily be obtained from the Commons history and I would have no objections if my overwrites were reverted in that case. Mike Turnbull (talk) 17:19, 22 April 2023 (UTC)
- Though perhaps the different image version should have a different name. Graeme Bartlett (talk) 12:10, 22 April 2023 (UTC)
- I have now redrawn the individual compounds Croconate blue, Croconate violet, 1,3-Bis(dicyanomethylene)squarate and 1,2-Bis(dicyanomethylene)squarate. Another I've changed is 2-(Dicyanomethylene)croconate. The oxocarbon article has lots of drawing that are consistent, although not aligned with usual conventions, and I've left that alone, along with peroxydicarbonate. Mike Turnbull (talk) 15:59, 21 April 2023 (UTC)
- I don't feel strongly about my diagrams in general. If someone wishes to redo them, please go ahead. But may I suggest better ways to spend that time. For example, last I checked there were dozens of fatty acid diagrams, used in dozens of articles, with the omega labels just wrong.
However, the new croconate diagrams, in particular, seem less informative than mine, and somewhat misleading. First, the convention that "unlabeled corners and ends are carbon atoms", while fully internalized by chemists, is quite confusing to a large section of the target public. Moreover, the new diagrams hide the aromatic character of the ring, and the "fractional" bonds and negative charges on the attached carbons that makes it work. (At least, that was the structure I gleaned from the papers I read.) The new image is just one of the "resonance" forms, isn't that so? And "resonance" is just a hack to fit the actual messy orbital truth into the old model of integer valences, localized integer charges, and binary bonds; isn't that so? For these reasons, shouldn't my diagrams (or others equivalent to them, e.g. 3D ball-and-stick) be kept as alternate representations, at least?
Finally, please note that uniformity of style and looks -- which is basically what the MOS is about -- has very little value for the reader. A commercial paper encyclopedia (dictionary, textbook, etc) must have strictly uniform style and typesetting, because otherwise the prospective buyer, leafing through it, would think that it is a mash-up of texts by disparate authors, randomly edited, with no plan and no oversight by a chief editor. But Wikipedia is a mash-up of texts by disparate authors, randomly edited, with no plan and no oversight by a chief editor. Thus, uniformizing its style and typesetting is downright dishonest: it leads the reader to think that Wikipedia is more reliable and complete than it actually is...
Methinks that the only "manual of style" that an editor needs to know is "look at a few other articles on related subjects, try to imitate what is good in them, and try to fix was is bad."
All the best, Jorge Stolfi (talk) 21:37, 24 April 2023 (UTC)- We chemists usually show the most representative resonance structure when possible (we can all agree that we aren't going to emphasize minority resonance structures). True, one needs significant knowledge to understand nuances of resonance structures. With the major resonance structure at least a reader can count electrons, check for conformance of trends in oxidation states and electron counting rules (octet rule, etc), and anticipate sites on a chemical that will be basic/nucleophilic vs acidic/electrophilic vs saturated/unsaturated. One cannot readily undertake those analyses with stylized images.
- In terms of "fully internalized by chemists, is quite confusing to a large section of the target public." Yes indeed. One needs a significant knowledge to read encyclopedia articles on specialized topics in chemistry. That is the way it is: Wikipedia presents facts. At the same time, we rely on discussions like this to review and perhaps recalibrate our communication styles. --Smokefoot (talk) 21:57, 24 April 2023 (UTC)
- Of course it is OK to use specialized jargon (textual or graphical) in those parts of articles that will be only of interest to specialists. However, the target readership of Wikipedia (or any general encyclopedia) definitely includes every non-specialist who read the term "geomagnetic jerk" or "neutrino oscillation" and wants to know what that is. Thus every article should strive to satisfy those readers, to the level of detail that will be useful to them, as well as the specialists. All the best, Jorge Stolfi (talk) 15:21, 25 April 2023 (UTC)
- @Jorge Stolfi You make some interesting points. My view is that no encyclopaedia, even Wikipedia, can comment on the complexity of aromaticity and resonance forms in every article in which it is relevant. We do so occasionally, as at the archetype, benzene, where the diagram I've placed here is included and does cover these issues.
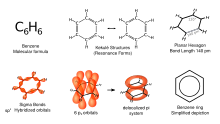
Benzene Representations - For better or worse, our MOS:CSDG specifies using a molecule editor and ACS drawing conventions. All these standard editors give Kekule-style representations of aromaticity and chemical drawings in Wikipedia mainly conform to these widespread standards also seen in Chemspider and Pubchem and virtually every professional chemical publication. My main concern with your drawings of the croconate examples, aside from the MOS, was that they didn't sufficiently emphasise that these are dianions: no charges are shown (see example below) and that outweighed the fact that they show the aromaticity better, albeit somewhat unconventionally.

Croconate violet, ACS conventions 
Croconate violet, Stolfi - However, I was certainly in error when I overwrote your files and that has now been undone. There may well be a case for expanding the Pseudo-oxocarbon anion article to use both types of diagram. Currently it has only mine. Mike Turnbull (talk) 11:46, 25 April 2023 (UTC)
- In terms of "fully internalized by chemists, is quite confusing to a large section of the target public." Yes indeed. One needs a significant knowledge to read encyclopedia articles on specialized topics in chemistry. That is the way it is: Wikipedia presents facts. At the same time, we rely on discussions like this to review and perhaps recalibrate our communication styles. --Smokefoot (talk) 21:57, 24 April 2023 (UTC)
Problem images
Jorge Stolfi I'm not sure if you are aware, but there are ways of flagging images that have issues on Commons (like these fatty acid diagrams). Sadly these don't yet feed into our Alerts list --Project Osprey (talk) 09:00, 25 April 2023 (UTC)
- Thanks! Jorge Stolfi (talk) 15:10, 25 April 2023 (UTC)
 Commons:WikiProject Chemistry/Deletion requests
Commons:WikiProject Chemistry/Deletion requests Commons:Category:Disputed chemical diagrams, /expired
Commons:Category:Disputed chemical diagrams, /expired Commons:Category:Low quality chemical diagrams, /expired
Commons:Category:Low quality chemical diagrams, /expired Commons:Category:Verified chemical structure diagrams
Commons:Category:Verified chemical structure diagrams Commons:Category:Media needing chemical classification
Commons:Category:Media needing chemical classification
Re: TOXNET has moved
(Continuation of Wikipedia talk:WikiProject Chemicals/Archive 2023#Re:_TOXNET_has_moved)
I just finished working on this. I moved {{HPD}} to {{CPID}} and updated the links on all pages that used it. Enjoy! — Garrett W. {☎ ✍} 18:54, 4 May 2023 (UTC)
Also just finished going through insource:/householdproducts\.nlm\.nih\.gov/ and updating all the broken links in those. — Garrett W. {☎ ✍} 20:30, 4 May 2023 (UTC)
- Thank you. What about deleting the redirect Template:HPD? --Leyo 22:23, 14 May 2023 (UTC)
- Sure, I could propose it for deletion.
- (edit) Done. — Garrett W. {☎ ✍} 06:14, 15 May 2023 (UTC)
Discussion at Wikipedia talk:WikiProject Medicine#Tea component theanine and its putative cognitive effects. SandyGeorgia (Talk) 12:40, 21 May 2023 (UTC)
Organofluorine article titles
I am trying to make sense of our articles on various classes of organofluorine compounds. We of course have organofluorine chemistry as the broad concept article, which is fine pursuant to WP:CHEMGROUP. But then we have Fluorocarbon, which at first seemed like it was a content fork and should be merged, analogous to chlorocarbon redirecting to organochlorine chemistry, but the focus of that article is really perfluorocarbon compounds, i.e., compounds with only C-F bonds. But, we also have perfluorinated compounds, which seems to focus on compounds again with C-F bonds in place of C-H bonds, but those also containing heteroatoms/functional groups. And then we have Hydrofluorocarbon, which are compounds with a C-F bond that retain at least one C-H bond.
If this seems like an appropriate way to separate out the content, then I propose moving fluorocarbon to perfluorocarbon, since the former term is too vague and should really redirect to the broader organofluorine chemistry article. But, that increases the confusion between perfluorocarbon and perfluorinated compounds. Hatnotes may be the answer there. Any thoughts? Mdewman6 (talk) 00:12, 27 May 2023 (UTC)
- I support the proposed reorganization. About 10 years ago, User:Shootbamboo, now inactive, created some fluorocarbon-related articles. The activity seemed to be motivated by that editor's interest in raising awareness of PFOS and related "forever chemicals" (which redirects to Per- and polyfluoroalkyl substances). One issue was whether organofluorine compound contained any C-H bonds or not. In any case, it seems timely to revisit the organization of our organofluorine subtopics.--Smokefoot (talk) 03:11, 27 May 2023 (UTC)
- The main reason to keep hydrofluorocarbon separate is because the important ones are refrigerants with less potential to damage the ozone layer. Perfluorocarbon and perfluorinated compounds are essentially the same thing: although it could be argued that the latter should include SF6 and UF6 etc. so maybe the title to keep should be the first one to emphasise that the article's scope is only within organic chemistry. I agree that organofluorine chemistry is the correct place for the main topic, covering everything that has at least one C-F bond. I'm not sure where PFAS should fit, though. PFOS is clearly a single substance (+ salts/esters) that merits its own article. Mike Turnbull (talk) 10:50, 27 May 2023 (UTC)
The article title Fluorocarbon is probably owed to the IUPAC Gold Book entry. Despite of that, moving this article to perfluorocarbon would make sense IMHO. BTW: The respective article in de.wikipedia is de:Perfluorcarbone. --Leyo 21:00, 27 May 2023 (UTC)
Good article reassessment for CS gas
CS gas has been nominated for a good article reassessment. If you are interested in the discussion, please participate by adding your comments to the reassessment page. If concerns are not addressed during the review period, the good article status may be removed from the article. Onegreatjoke (talk) 01:07, 28 May 2023 (UTC)
Quinuclidine derivs
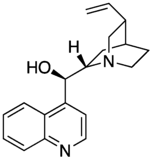
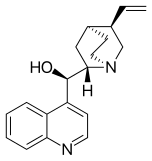
Maybe a minor point but I have noticed that almost all of the cinchona alkaloids (quinine etc) are or were depicted with the quinuclidine group drawn with one skewed (CH2)2 backbone. The thing about quinuclidine is that it is rigid and all-chair.--Smokefoot (talk) 14:23, 27 May 2023 (UTC)
- A likely explanation is that the misleading form is much easier to draw. If you wish to improve and replace the existing images then please go ahead - but I expect the situation might be replicated in the literature. The two forms are sufficiently different to the eye that some readers might struggle to reconcile the depictions. Would there be any merit to adding a note somewhere on the page? Project Osprey (talk) 18:58, 27 May 2023 (UTC)
- Good points. It does seem that the drawing on the left captures the correct geometry however. And adding a remark on the page that the CH2's are eclipsed might be a good idea. --Smokefoot (talk) 19:44, 27 May 2023 (UTC)
- One of the reasons that professional organic chemists are likely to use the "misleading" structure is that it is the only one correctly recognised by chemical drawing packages and conforms to the "rules" originally set out by MDL Information Systems and implemented in their molfile format, still widely used today. Perspective drawings involve drawing at least one bond with a gap (as seen in "better") or one ends up with an ambiguous image illusion. Both drawings have their place, depending on context. Mike Turnbull (talk) 16:50, 31 May 2023 (UTC)
- Good points. It does seem that the drawing on the left captures the correct geometry however. And adding a remark on the page that the CH2's are eclipsed might be a good idea. --Smokefoot (talk) 19:44, 27 May 2023 (UTC)
Shall synthesis routes to various end products via the same intermediate be counted as routes to that intermediate?
Hello, I would like to account for methyl nitrite. It was an intermediate product in a synthesis of dimethyl oxalate. Including that synthesis, shall all routes via methyl nitrite be counted as routes to methyl nitrite? According to a previous discussion, yes it shall:
Any intermediate in an industrial process is a commercially important chemical in its own right, -- DMacks (talk) 15:15, 23 July 2017 (UTC)
-- Ktsquare (talk) 01:30, 8 June 2023 (UTC)
Help and mediation needed at Eucalyptol
I am involved in a revert battle at eucalyptol and the issue requires mediation because we are just going back and forth. It would be nice to resolve this struggle within the chemistry project. My case at least is going to be stated on Talk:Eucalyptol. --Smokefoot (talk) 13:08, 10 June 2023 (UTC)
Sulfur dioxide and climate change pages related to it
Hello all! I normally participate over in Wikipedia:WikiProject Climate Change, but there is one matter which overlaps substantially with the scope of this project.
Essentially, I think you all know that sulfur dioxide has a reflective, anti-greenhouse effect in the atmosphere. This makes it a popular subject for all sorts of speculation about ways to address warming and even the "true" extent of current warming, and this speculation has produced a lot of Wikipedia articles with a lot of overlap as well. By my count, we currently have:
They all have substantial overlap with each other. Some of it is justified (i.e. stratospheric aerosol injection is technically only one of several possible ways to do solar geoengineering, even if it is by far the most practical one), some not so much, and altogether, managing so many overlapping pages is not practical, so they weren't really managed. As I discovered earlier this year, pretty much all of them were out of date - sometimes by whole decades, with most of citations from 2000s. Considering that this is a subject of active interest, with some of these pages consistently scoring hundreds views per day, this is hardly an idle concern.
I have more-or-less managed to get global dimming up to date by now, and at least managed to update the lead and first paragraphs of solar geoengineering (the rest is still a semi-active battleground of active claims and counter-claims about competing proposals that'll take a while to untangle). However, I do not see much point in retaining a stratospheric sulfur aerosols page, when all it provides is very general (and currently very dated) information, some of which belongs in [stratospheric aerosol injection, some in global dimming, and some can most likely just be folded back into sulfur dioxide article. Sulfate aerosols is likely not needed for similar reasons.
What would you say about this proposal? I raised it on WP:ClimateChange earlier, but it unfortunately doesn't have enough editors to discuss every single matter in detail. I hope this is sufficiently close to the project's scope to be discussed here. InformationToKnowledge (talk) 19:55, 21 June 2023 (UTC)
- Well, with regards to " I think you all know that sulfur dioxide has a reflective, anti-greenhouse effect in the atmosphere." Huh? This is a group of chemists. Reflectivity is hardly of interest in the chemistry world. But more to the point, my experience is that controversial topics like water fluoridation spawn many articles on related topics. Without speculating on why this spawning and splitting arises, it is undesirable because curation of the articles is awkward and all sorts of fringe perspectives slip in. So, assuming that you are not a jerk and science denialist, you should consolidate those articles. It could be a major service to our readership.--Smokefoot (talk) 20:29, 21 June 2023 (UTC)
- I will add that sulfur dioxide in itself would not be "reflective" and is probably a greenhouse gas in itself, as it would have additional absorption in the infrared. However once it is oxidised to sulfur trioxide, it would nucleate cloud droplets, and then have the properties you say. If you think a couple of articles are unneeded then you can merge the content, and turn them into redirects in the appropriate sections. Graeme Bartlett (talk) 00:28, 22 June 2023 (UTC)
- Since you have agreed with the notion, and no-one else had objected, it is now done. Sulfate aerosols and [Stratospheric sulfur aerosols]] have been merged into the closest appropriate articles, and a lot of information had been streamlined (not to mention better referenced and illustrated.) InformationToKnowledge (talk) 17:22, 28 June 2023 (UTC)
What do we think about Guidechem.com citations?
Recently a variety of far-eastern IP addresses have been adding plausible-sounding sentences to chemical articles, with citations to guidechem.com, so that as of today this insource search gives 54 hits. A few others have already been reverted by Project Osprey, e.g. here today, MadeOfAtoms e.g. here today and myself. The website to which all these link is not one on our list of chemical databases but if its homepage here is to be believed it lists over 9 million chemicals from 30,000+ suppliers and its MSDS such as this one for serotonin are nearly as comprehensive as the corresponding pubchem entry. Indeed, guidechem.com may have used pubchem as their source. Clearly guidechem.com is not a reliable source as normally defined since it is a database with only a few named sources. It has some interesting quirks, such as (from the serotonin "other information") Serotonin is the baby boomer of neurotransmitters....
. What should our attitude to this website be? Mike Turnbull (talk) 11:32, 29 June 2023 (UTC)
- I wasn't aware there was a pattern. Perhaps this is an attempt at Search engine optimization, by getting Wikipedia to cite guidechem.com --Project Osprey (talk) 12:01, 29 June 2023 (UTC)
- Incidentally, that quotation comes directly from this chapter in a book. Mike Turnbull (talk) 12:05, 29 June 2023 (UTC)
- I did not add that site to the list of chemical databases as I considered it to be a marketer. For the same reason I did not add chemical suppliers or manufacturers. But if someone else added it to that list, I would not remove it. As a reference it would be inferior, and better to cite something closer to where the information came from, even a manufacturer. Graeme Bartlett (talk) 21:56, 29 June 2023 (UTC)
- This website shows info that is mostly copy-pasted from what seem to be reliable sources, usually without attribution. For example the pentane edit adds text that was copy-pasted from Patty's Toxicology (2012), without attribution. One could consider this to be useful info in the pentane case, but it would need a link to the proper source and paraphrasing to avoid copyvio. Seems worse than useless given the info on pubchem. –MadeOfAtoms (talk) 22:28, 29 June 2023 (UTC)
- A few guidechem additions popped up on my watchlist lately, not even written coherently sometimes. Clearly spam IMO. Nuke it all. DMacks (talk) 05:18, 6 July 2023 (UTC)
- I've now 31h-blocked 42.98.200.240, and will not hesitate to block others based on this seeming consensus here. DMacks (talk) 05:22, 6 July 2023 (UTC)
- That were flagged by WikiProject Spam, including cross-wiki to multiple languages, in 2011! We also had a discussion here at that time about blacklisting it (Wikipedia talk:WikiProject Chemicals/Archive 2011#Blacklisting http://www.guidechem.com/), involving 4 admins at least somewhat supporting doing so. DMacks (talk) 05:29, 6 July 2023 (UTC)
- Thanks User:MadeOfAtoms for helping to clean up the uses. A bunch of named accounts are now continuning this behavior across multiple-wikis and are getting g-locked for spam. I filed an enwiki blacklist request, no prejudice against upgrading it to global blacklist. DMacks (talk) 16:37, 8 July 2023 (UTC)
- Thanks, @DMacks for the archive discussion and your other actions. MadeOfAtoms has done a good job of the cleanup so my insource search now shows just 12 instances. I'll help with removing the rest of these and any new ones that appear. Mike Turnbull (talk) 17:31, 8 July 2023 (UTC)
- Thank you for blocking and cleaning, and I'm happy to help out. –MadeOfAtoms (talk) 17:59, 8 July 2023 (UTC)
- I just removed the last handful from mainspace. DMacks (talk) 18:28, 9 July 2023 (UTC)
- Thanks, @DMacks for the archive discussion and your other actions. MadeOfAtoms has done a good job of the cleanup so my insource search now shows just 12 instances. I'll help with removing the rest of these and any new ones that appear. Mike Turnbull (talk) 17:31, 8 July 2023 (UTC)
Silylgermane
I'm currently at in impasse with user:Bernardirfan over the fate of Silylgermane. I think the page should be deleted, they feel otherwise. For my part I think the compound fails our usual notability guidelines. It is produced by mixing two highly toxic and phyrophic gasses, Germane and Silane, both of which are hugely important in semiconductor synthesis. I think silylgermane was produced to answer the question of "what happens if you combine them" - but beyond this I can find no actual application. It's just a bit of chemical esoterica. Many current references are below our usual standard. I've decided to bring the matter here rather than Pages for Deletion as I suspect technical rather than procedural arguments will decide the outcome. Project Osprey (talk) 20:29, 16 July 2023 (UTC)
- There are many articles about certain chemical compounds that have no application at all, they are just an esoteric compounds, even non-existent compounds, which are just of academic interest and for scientific research, all across Wikipedia, yet, they are not deleted. I would feel discriminated if my article Silylgermane was rather deleted, than kept for future improvement. But let's wait the consensus to decide the fate of my article. Bernardirfan (talk) 22:47, 16 July 2023 (UTC)
- For weird reasons, I am kinda into germane, and I can say that H3SiGeH3 is real and well characterized. The early paper by (Nobelist) MacDiarmid is shaky, agreed. There are a lot of theory papers, but they will calculate anything it seems. One team really nailed this stuff though. Now about notability, that is a tougher question. Some of us (me) have added all sorts of esoteric compounds to this project, so I am not a good judge. In any case, here is a primary ref to silylgermane doi 10.1021/ja051411o. The compound is mentioned (only) in a recent review as follows "Extended tetrelane hydrides containing both Si and Ge centers (e.g., the butane analogue H3Ge-SiH2–SiH2–GeH3) have been developed for CVD ..." "Molecular Main Group Metal Hydrides". Chem. Rev. 121: 12784–12965. 2021. doi:10.1021/acs.chemrev.1c00278.
{{cite journal}}: Unknown parameter|authors=ignored (help). --Smokefoot (talk) 23:05, 16 July 2023 (UTC)- In Bernardirfan's defence, I think I might just be bias again hydrides heavier than Calcium. They seem to occupy some weirdly privileged position as automatically valid topics for articles - when in practice they are often utterly impractical to make, with few (often disagreeing) reports of their properties and no practical applications.
- I don't for one moment doubt that this stuff exists. Anyone who can do this sort of chemistry (without resorting to picking bits of burning glass out of their face) must be a good chemist. Existence by-itself is not sufficient for notability. I'm fine with esoteric when it's interesting - but this stuff is exactly what you would expect. Structure and physical properties (bpt) are about midway between Disilane and Digermane. Tedious. Project Osprey (talk) 00:07, 17 July 2023 (UTC)
- Then why articles about the compound Gold heptafluoride, which has no practical applications at all, and even non-existing compounds like Osmium octafluoride and Xenon octafluoride and many, many more similar articles across Wikipedia, writed by many editors, are notable? I consider them notable too. By the way, please, do not delete all those articles, because Wikipedia would be truncated by deleting a huge amount of important, notable information and knowledge. I think the user:Project Osprey might just be biased against hydrides heavier than Calcium hydride. But I am not biased against them, I am open minded for any esoteric and hypothetical compounds. Even hypothetical compounds are notable according to me and other editors who obviously approved all those articles about those compounds. Many structural and physical properties of Hexadecane are about midway between Tetradecane and Octadecane, and some structural and physical properties of Stannane are about midway between Germane and Plumbane, just as some structural and physical properties of Silylgermane are about midway between Disilane and Digermane, so what, other editors and me don't see any problem about all those midway articles. All those compounds are still notable since they are obviously approved (do not delete them too). Bernardirfan (talk) 00:44, 17 July 2023 (UTC)
- Patrol approval does not necessarily mean that the topic really is notable. Please be wary of hypothetical compounds. AFD is still possible. But I prefer to have articles rather than delete them, so it's mainly the misleading articles that I will want to eliminate. Currently there is an outbreak or actinide oxyhalide articles. But the substances do seem to exist even if very unimportant. What I hope is that the next big important advance in materials or chemicals will already have an article on Wikipedia. Graeme Bartlett (talk) 10:03, 17 July 2023 (UTC)
- Thank you. Bernardirfan (talk) 10:10, 17 July 2023 (UTC)
- Patrol approval does not necessarily mean that the topic really is notable. Please be wary of hypothetical compounds. AFD is still possible. But I prefer to have articles rather than delete them, so it's mainly the misleading articles that I will want to eliminate. Currently there is an outbreak or actinide oxyhalide articles. But the substances do seem to exist even if very unimportant. What I hope is that the next big important advance in materials or chemicals will already have an article on Wikipedia. Graeme Bartlett (talk) 10:03, 17 July 2023 (UTC)
- Then why articles about the compound Gold heptafluoride, which has no practical applications at all, and even non-existing compounds like Osmium octafluoride and Xenon octafluoride and many, many more similar articles across Wikipedia, writed by many editors, are notable? I consider them notable too. By the way, please, do not delete all those articles, because Wikipedia would be truncated by deleting a huge amount of important, notable information and knowledge. I think the user:Project Osprey might just be biased against hydrides heavier than Calcium hydride. But I am not biased against them, I am open minded for any esoteric and hypothetical compounds. Even hypothetical compounds are notable according to me and other editors who obviously approved all those articles about those compounds. Many structural and physical properties of Hexadecane are about midway between Tetradecane and Octadecane, and some structural and physical properties of Stannane are about midway between Germane and Plumbane, just as some structural and physical properties of Silylgermane are about midway between Disilane and Digermane, so what, other editors and me don't see any problem about all those midway articles. All those compounds are still notable since they are obviously approved (do not delete them too). Bernardirfan (talk) 00:44, 17 July 2023 (UTC)
- For weird reasons, I am kinda into germane, and I can say that H3SiGeH3 is real and well characterized. The early paper by (Nobelist) MacDiarmid is shaky, agreed. There are a lot of theory papers, but they will calculate anything it seems. One team really nailed this stuff though. Now about notability, that is a tougher question. Some of us (me) have added all sorts of esoteric compounds to this project, so I am not a good judge. In any case, here is a primary ref to silylgermane doi 10.1021/ja051411o. The compound is mentioned (only) in a recent review as follows "Extended tetrelane hydrides containing both Si and Ge centers (e.g., the butane analogue H3Ge-SiH2–SiH2–GeH3) have been developed for CVD ..." "Molecular Main Group Metal Hydrides". Chem. Rev. 121: 12784–12965. 2021. doi:10.1021/acs.chemrev.1c00278.
- I reckon that Silylgermane is notable based on two references that have substantial content. However the article has several lower quality database references that could be improved. Graeme Bartlett (talk) 23:20, 16 July 2023 (UTC)
- Some articles of questionable notability are poorly written or thinly supported. If these editors really want to help the project they could contribute to widely read topics, say, on polymers or chemicals in everyday life (boring white solids). Although these weakly notable articles seem harmless (and maybe they are), one problem is that these editors get invigorated and keep at it. So we get more and more such cruft. Another problem is that these often questionable articles do not reflect well on Wiki Chem. IMHO.--Smokefoot (talk) 01:50, 17 July 2023 (UTC)
Journal links that go to another vendor
When editing Claisen condensation, the following refs were encountered:
- Claisen, L. (1887). "Ueber die Einführung von Säureradicalen in Ketone". Berichte der Deutschen Chemischen Gesellschaft. 20 (1): 655–657. doi:10.1002/cber.188702001150.
- Hauser, C. R.; Hudson, B. E. Jr. (1942). "The Acetoacetic Ester Condensation and Certain Related Reactions". Organic Reactions. 1: 266–302. doi:10.1002/0471264180.or001.09. ISBN 0471264180.
The URL's direct to zenodo.org and www.scribd.com, which seems undesirable because we are promoting an intermediate vendor. But maybe these sites are useful by providing access to articles that would otherwise be behind a paywall. I also increasingly see refs that link to a ResearchGate's URL. I have been removing these links to Research Gate because they do not seem to offer any advantage vs the journal URL, but maybe I shouldn't. Comments are welcome.--Smokefoot (talk) 14:34, 9 July 2023 (UTC)
- I agree that we should not be generally preferring any intermediate vendor, and the DOI (or similar token) leads to the publisher already. Therefore, we should not have direct URL links to the publisher's site (this is already against citation guidelines). However, if there is a valid third-party archive it might be useable if it does not violate the license of the content. Scribd in particular is a problem because it's essentially a free upload site, and therefore ripe for copyright-violations--it is a violation of WP sitewide guidelines to link to such things. Specifically, the Organic Reactions ref is a 1942 publication and therefore typically still protected by copyright, so that link is not acceptable. Zenodo is more careful with the content it hosts. Specifically, the Berichte ref is an 1887 publication, and therefore no longer protected by copyright (public domain due to age), so it is not a violation to link to a copy of it. The publisher's own site (via DOI) paywalls it, so there is added value to linking to the Zenodo archive (it correctly identifies the content as "open access"). DMacks (talk) 17:40, 9 July 2023 (UTC)
- The Royal Society of Chemistry in the UK has recently partnered with ResearchGate to make their open-access journals more accessible. So links to that resource are going to become more frequently used. For articles with DOI that link to a paywall, any ResearchGate URL offering open access should be welcomed. That said, I assume that the RSC's own OA journals will be accessible from the DOI directly, so an additional ResearchGate link seems unnecessary. Mike Turnbull (talk) 10:37, 17 July 2023 (UTC)
FAR
I have nominated Hydrochloric acid for a featured article review here. Please join the discussion on whether this article meets the featured article criteria. Articles are typically reviewed for two weeks. If substantial concerns are not addressed during the review period, the article will be moved to the Featured Article Removal Candidates list for a further period, where editors may declare "Keep" or "Delist" in regards to the article's featured status. The instructions for the review process are here. Keres🌕Luna edits! 16:39, 25 July 2023 (UTC)
What about moving back this stub into the main space? Does anyone have access to SciFinder to look for a CAS RN? Leyo 12:48, 3 August 2023 (UTC)
- It's on Chemsider here (InChIKey=SXDHPHPRCINSFL-XUQYBVARSA-P) but they don't quote a CAS number. Chemspider has another drawing as a neutral but even that doesn't have a RN (InChIKey=SXDHPHPRCINSFL-XUQYBVARSA-N). I'm in doubt as to whether is stuff is really notable enough to warrant an article. The InChIKey leads to one patent hit for US20040229803 but that was abandoned more-or-less as soon as it was published. Pubchem doesn't come up with much. Mike Turnbull (talk) 13:39, 3 August 2023 (UTC)
- CAS 172998-23-1, SciFinder shows a total of 4 refs. --Project Osprey (talk) 15:03, 3 August 2023 (UTC)
- Thank you. --Leyo 00:38, 4 August 2023 (UTC)
- CAS 172998-23-1, SciFinder shows a total of 4 refs. --Project Osprey (talk) 15:03, 3 August 2023 (UTC)
Credibility bot
As this is a highly active WikiProject, I would like to introduce you to Credibility bot. This is a bot that makes it easier to track source usage across articles through automated reports and alerts. We piloted this approach at Wikipedia:Vaccine safety and we want to offer it to any subject area or domain. We need your support to demonstrate demand for this toolkit. If you have a desire for this functionality, or would like to leave other feedback, please endorse the tool or comment at WP:CREDBOT. Thanks! Harej (talk) 18:06, 5 August 2023 (UTC)
Fats vs fatty acids
Merging of polyunsaturated fatty acids and polyunsaturated fats might be a well intentioned, but it is a dumb or at least naive idea. Fatty acids are fatty acids, fats are triglyberides and related materials. What next? See Talk:Polyunsaturated fat. Can someone with time and skills please undo this mess brought on by user:Amakuru. --Smokefoot (talk) 21:10, 5 August 2023 (UTC)
- @Smokefoot: I literally just told you I did not "bring on the mess". I did not do that merge. If you want it reverted, I'll be happy to do so, but please stop casting WP:ASPERSIONS and assuming bad faith. Thanks — Amakuru (talk) 21:11, 5 August 2023 (UTC)
Chembox or DrugBox mismatch
I came across this article (Sinclair, Gabriel; Thillainadarajah, Inthirany; Meyer, Brian; Samano, Vicente; Sivasupramaniam, Sakuntala; Adams, Linda; Willighagen, Egon L.; Richard, Ann M.; Walker, Martin; Williams, Antony J. (24 October 2022). "Wikipedia on the CompTox Chemicals Dashboard: Connecting Resources to Enrich Public Chemical Data". Journal of Chemical Information and Modeling. 62 (20): 4888–4905. doi:10.1021/acs.jcim.2c00886.) which is worth looking at for those in our sub-project. It raises a number of issues with some of our articles, which appear not to be sorted out yet. For example what is the connection between JWH-210 and [[]JWH-182]]? or why does JWH-203 include CAS no for JWH-204? But some were fixed, eg user:ChemConnector changed smiles on JWH-359. It appears that articles with multiple substances, may be indicated by multiple infoboxes or indexing, on non-indexed multiple strings or RNs cause trouble matching things up. So what can we do to make the Wikipedia data more useful and accessible for large database matching? Graeme Bartlett (talk) 02:33, 19 June 2023 (UTC)
- Somewhat related: if you go to this resource [2] and select 'Browse Errors' you see a list of all pages with SMILES errors, chemboxes without SMILES and duplicated SMILES. Handy for error checking. --Project Osprey (talk) 09:55, 19 June 2023 (UTC)
- Thanks, that list is giving me more SMILES values to fix. Graeme Bartlett (talk) 07:51, 20 June 2023 (UTC)
- As you'll know, some of the authors of that article are active Wikipedia editors. One thing that has been done since it was published is that the DTXSID (and ECHA Infocard) values are not maintained in the Chembox/Drugbox but instead are maintained centrally in Wikidata. The linkage between Wikidata's QID and external IDs makes it more likely the correct one-to-one relationship can be maintained. I'd be happy to help out fix any ongoing issues but I think we need guidance from Walkerma etc. as to the current priorities. Mike Turnbull (talk) 10:58, 19 June 2023 (UTC)
- I'm travelling at the moment (actually at a Romanian airport right now), so it's hard for me to follow through right now, but I'm sure we could set something up to fix things systematically once I get home. The main coordinator of the project is user:ChemConnector, but other co-authors are more familiar with Wikidata and should probably get involved. Thanks, Walkerma (talk) 08:56, 21 June 2023 (UTC)
- Just to give an hint regarding the current discussion in WD about identification of molecules. If WD solved the question of stereisomers and position isomers (every different form has its own item), the question for zwitterions and tautomers is still open. This affects the generations of InChI/InChIKey/SMILES reprsentations and therfore the correct identification in the databases. Another welle frequent problem is the definition of covalent/ionic bond for salts and complexes. Current discussion to solve these problems is available here. Snipre (talk) 12:17, 14 August 2023 (UTC)
Requested move at Talk:Β-Butyrolactone#Requested move 16 August 2023

There is a requested move discussion at Talk:Β-Butyrolactone#Requested move 16 August 2023 that may be of interest to members of this WikiProject. ModernDayTrilobite (talk • contribs) 16:06, 25 August 2023 (UTC)
Is www.americanelements.com reliable?
See https://www.americanelements.com/manganese-ii-iodide-7790-33-2. Yes, I see that they are open access (what vendor isn't?) But I dont see why they are a source of reliable information.--Smokefoot (talk) 12:42, 20 September 2023 (UTC)
- We have 214 citatons to it currently and it is mentioned at Wikibooks as a source. Their website looks very professional but they don't, as far as I can tell, say where they get their information from. Mike Turnbull (talk) 13:03, 20 September 2023 (UTC)
- They have out of date safety statements. So it may not have been updated for decades. Probably their info is from PubChem. They would want correct info to put in their MSDS or SDS. For physical properties it would be better to use a source that can trace back its info to the ultimate primary sources. Graeme Bartlett (talk) 21:19, 20 September 2023 (UTC)
Merge discussion of Metirosine
A case where we have separate articles for an enantiomer and the racemate, but they are written like duplicate articles. Please join the discussion at Talk:Α-Methyl-p-tyrosine#Merge_discussion to decide how best to handle this. Thanks! Mdewman6 (talk) 22:06, 20 September 2023 (UTC)
Proposed deletion of Geissolosimine and Interiotherin
Hi! The articles on Geissolosimine and Interiotherin have been proposed for deletion because they are orphans and have been for ten years. Can someone from the chemicals project have a look please? And if you decide to remove the proposed deletion template, could you also please de-orphan the articles?~~~~ Ruud Buitelaar (talk) 23:50, 17 September 2023 (UTC)
- Orphan is no reason to delete. Graeme Bartlett (talk) 22:45, 21 September 2023 (UTC)
Classify molecular formula set index articles as list or disambiguation pages?
Hello! I going through the many list / disambiguation pages that fall under the WPChemicals umbrella. A good number of these fall under Category:Molecular formula set index articles, and only have a handful of entries on each page, which is justifiable in this situation. However, what I wanted to ask about is "how should these articles be categorized inside the wikiproject?" When going through these talk pages, there seem to be many different "project-category outcomes" that each of these articles fall into, seemingly arbitrarily. Some of the set index articles are listed as part of the WikiProject, yet may contain articles that are not part of the WikiProject. The vice versa is also true; some of the articles are NOT a part of the project, while all of the indices on the page are. But, this is kind of getting ahead of the program. The main question is which outcome would be most correct with dealing with these set index articles?
The status quo is all over the board, but just perusing for a bit led to the following categorization of common outcomes for set index articles (each of which contain articles that are all part of WPChemicals):
In WikiProject Chemicals:
- List class / Low importance (and project lists & index) = Talk:C3H7I, Talk:C4H2, Talk:C5H9NO3S, Talk:C8H18N4O2
- List class / Low importance (and project lists) = Talk:C4H10S, Talk:C7H6O6, Talk:C9H11FN2O5, Talk:C11H10N2O2, Talk:N4O
- List class / Low importance (no project lists) = Talk:C2H2, Talk:C4F8, Talk:C5H11Cl, Talk:C9H12N2O6, Talk:C11H15ClO2
- Dab class / Low importance (and project dab) = Talk:BrO, Talk:C6H14O3, Talk:C7H15Cl2N2O4P
- Dab class / Low importance (no project dab) = Talk:C3H3NS, Talk:C21H34O3
- Dab class / Low importance (no project dab, yet has project lists for some reason) = Talk:C2Cl3F3
- Dab class / NA importance (and project dab) = Talk:C4F6, Talk:C8H10N2O3S
- Dab class / NA importance (no project dab) = Talk:C8H5NO6, Talk:C11H17BrNO2
Not in WikiProject Chemicals:
- Just in project dab = Talk:C7H7NO, Talk:C8H6O, Talk:C9H17N, Talk:C10H8O, Talk:C10H15NO3
- No talk page entirely = Talk:C10H10O5, Talk:O2+
Going through this category was a strange experience, as its seemingly possible to click on any molecular formula index article, and be given a surprise box of wikiprojects on its talk page. Because there's a couple thousand or so of similar pages, it may be worth using a bot or script to go through and align the appropriate set index articles for WikiProject Chemicals, and/or any other concerned WikiProjects (Disambig, Lists, etc). The first thing though, I think, is figuring out the appropriate way to deal with these. Utopes (talk / cont) 19:05, 8 September 2023 (UTC)
- That activity may be in the category of busy work, and not so useful to our readers. It may be useful to us though. I think we should have them as class=disambig. But set indexes do not have to link to existing articles and may also include totally non-notable substances. But if we do include those chemicals or imaginary chemicals we may need to start referencing to show that the item exists or has been studied, as there is no article with references linked. Unless we are members of the disambig or lists project we should leave that tagging alone. Graeme Bartlett (talk) 22:52, 21 September 2023 (UTC)
Hi all,
It's been a while and I hope everybody's been well. I'm not active anymore, but the recent renaming of the above two articles to terminal alkene and straight-chain terminal alkene caught my eye.
To this chemist, alpha-olefins, linear alpha-olefins, and polyalphaolefins are articles of commerce useful in making polyethylene, detergents, lubricants, and other materials. Terminal alkenes refer to the specific moiety in scientific literature. Not quite the same thing.
Could I please ask this community to review the change? Thanks! --Rifleman 82 (talk) 16:13, 15 October 2023 (UTC)
- The organic books I have (March's 2020 and Clayden 2001) largely ignore *olefin and redirect to *alkene in the index, like the texts that Mdewman6 has. To get a rough sense of usage, Google Scholar gives 1.5 times as many results for "alpha[α][-]olefin[s]" as for "terminal alkene[s]". The ratio decreases to 1.35 when looking only at the past decade, so there's a gradual trend toward terminal alkenes. General Google search gives twice as many alpha-olefins as terminal alkenes, and googling with Bing gives similar results. Maybe industrial usage favors olefins but I'd say there's no clear preference based on WP:COMMONNAME or on overall technical usage. –MadeOfAtoms (talk) 23:21, 15 October 2023 (UTC)
- A literal reading of the both phrases suggests equivalence. I contend that alpha-olefins is a term of art referring to the low MW hydrocarbon, whereas terminal alkenes have a more general meaning that is not restricted to this (very significant) class of industrial products. These low MW materials are largely mature products, so you probably won't see many recent references in Google Scholar. Regardless, I do believe the distinction emerges as you look at the results. As an example, the first 5 hits for each:
- Alpha olefin
- Recent findings and experiences with alpha olefin sulfonates
- Foam films stabilized with alpha olefin sulfonate (AOS)
- Performance and anti-wear mechanism of CaCO3 nanoparticles as a green additive in poly-alpha-olefin
- [BOOK] Tailor-made polymers: via immobilization of alpha-olefin polymerization catalysts
- [PDF] Model Ziegler-Natta. alpha.-Olefin Polymerization Catalysts Derived from [{(. eta. 5-C5Me4) SiMe2 (. eta. 1-NCMe3)}(PMe3) Sc (. mu. 2-H)] 2 and [{(. eta. 5 …
- Terminal alkene
- Terminal Olefin (1-Alkene) Biosynthesis by a Novel P450 Fatty Acid Decarboxylase from Jeotgalicoccus Species
- [PDF] Metabolic activation of valproic acid and drug-mediated hepatotoxicity. Role of the terminal olefin, 2-n-propyl-4-pentenoic acid
- Selective dihydroxylation of non-conjugated dienes in favor of the terminal olefin
- Selective Isomerization of a Terminal Olefin Catalyzed by a Ruthenium Complex: The Synthesis of Indoles through Ring‐Closing Metathesis
- High‐Yielding Tandem Hydroformylation/Hydrogenation of a Terminal Olefin to Produce a Linear Alcohol Using a Rh/Ru Dual Catalyst System
--Rifleman 82 (talk) 01:49, 16 October 2023 (UTC)
- Branched chain terminal alkenes are old and often poorly defined. During WWII massive production capacity was developed to provide high-octane fuel for fighter aircraft (see Vladimir Ipatieff). They were often things like 'propylene tetramer', although the name is only loosely accurate. After the war the businesses needed to find a use for these things and they ended up getting used in the developing field of synthetic detergents, amongst others. Branched chains have poor biodegradability and often toxicity issues, so they've been increasingly replaced since the 60s with linear alpha olefins. In my mind the two are closely related. --Project Osprey (talk) 13:52, 16 October 2023 (UTC)
- I recommend that we merge linear alpha olefin into alpha-olefin, which is common jargon. After the merger (the hard part), we consider other names like terminal alkene.--Smokefoot (talk) 14:15, 16 October 2023 (UTC)
- alpha-olefin is currently a redirect. Do you mean to merge linear alpha olefin into terminal alkene? I do agree the two articles should be merged. Mdewman6 (talk) 02:41, 19 October 2023 (UTC)
- Oh, good point (sorry), yes, merge linear alpha olefin into terminal alkene. --Smokefoot (talk) 13:29, 19 October 2023 (UTC)
- alpha-olefin is currently a redirect. Do you mean to merge linear alpha olefin into terminal alkene? I do agree the two articles should be merged. Mdewman6 (talk) 02:41, 19 October 2023 (UTC)
- I recommend that we merge linear alpha olefin into alpha-olefin, which is common jargon. After the merger (the hard part), we consider other names like terminal alkene.--Smokefoot (talk) 14:15, 16 October 2023 (UTC)
- Branched chain terminal alkenes are old and often poorly defined. During WWII massive production capacity was developed to provide high-octane fuel for fighter aircraft (see Vladimir Ipatieff). They were often things like 'propylene tetramer', although the name is only loosely accurate. After the war the businesses needed to find a use for these things and they ended up getting used in the developing field of synthetic detergents, amongst others. Branched chains have poor biodegradability and often toxicity issues, so they've been increasingly replaced since the 60s with linear alpha olefins. In my mind the two are closely related. --Project Osprey (talk) 13:52, 16 October 2023 (UTC)
Should articles such as Trimethylbenzenes and Cymenes have singular or plural titles?
From WP:CHEMGROUP: "For groups of compounds named after a simple parent compound, articles about the group should be located at the plural of the parent compound name, e.g. hydrazines, silanes, boranes, diphosphenes." The title "Silane" can be ambiguous because it can either mean the compound Silane or the group of compounds called Silanes, so there has to be two articles with a singular and a plural title. There is also a difference between Phenolate and Phenolates, since "phenolate" refers to the anion, while "phenolates" refers to the group of compounds containing the "phenolate" anion. However in the case of cymene, there is no singular parent compound called "cymene", only three different compounds p-Cymene, o-Cymene, and m-Cymene, so there is no ambiguity with the title Cymene which can only refer to a group of compounds or a mixture of isomers, so I think Cymenes should be moved to Cymene. Same with moving Trimethylbenzenes, Diisopropylbenzenes, and Tetramethylbenzenes to their respective singular titles. In general when there is no possible ambiguity, articles about chemical groups are always singular titled, e.g. Chlorotoluene. Michael7604 (talk) 16:34, 19 October 2023 (UTC)
- Comment: A move request to determine the answer to this concern, as well as possibly establish precedence for this concern, is being discussed at Talk:Diisopropylbenzenes#Requested move 19 October 2023. Steel1943 (talk) 17:22, 19 October 2023 (UTC)
- Comment. Yes, these pages should have singular titles, because they only discuss isomers of the simple compound, not derivatives of it. They are essentially beefed-up version of chemindices, e.g. Dichlorobenzene, where the isomers are described and linked. Also compare to Cresol, Anisole, etc.. I agree there should be clarification at WP:CHEMGROUP about this distinction; I will amend it once we have consensus here. Mdewman6 (talk) 17:57, 19 October 2023 (UTC)
- Plural. WP:CHEMGROUP's advice is good. We are chemists, so we know that cymene is not a chemical compound and the article will probably refer to a set of three materials. Most casual readers won't know this and hence they will be assisted by using the plural title. The same principle is used in Wikimedia's categorisation system. It has both "pyridine" and "pyridines": the former for the specific and the latter for the general. (I am aware that this goes against the conclusion at Talk:Diisopropylbenzene#Requested_move_19_October_2023 but that was not a page I had on my watchlist.) Mike Turnbull (talk) 11:14, 1 November 2023 (UTC)
Is the article copper salicylate about copper(I) salicylate or copper(II) salicylate? The infobox has data mostly for the copper(I) compound, but also for copper(II) salicylate and for a cationic form (PubChem link). The statements about chemical structure in the text (and the cited reference) refer to copper(II) salicylate. The cited reference may be inconsistent as well - I don't have access to the full text of the article, so I'm not sure. Copper(II) salicylate used to be a stub of an article, but has been redirected to salicylic acid. Can someone have a look? Marbletan (talk) 19:17, 31 October 2023 (UTC)
- thank you for catching this problem. The article should probably be about the Cu(II) derivative since copper(II) carboxylates are far more prevalent than the Cu(I) derivatives. It would be a blue solid. Let me do some checking and fix this.--Smokefoot (talk) 21:52, 31 October 2023 (UTC)
- In 2019 Edgar181 changed this from a disalicylate to the mono. In all the early history there was no reference. I added a referenced statement, but that was for a basic copper(II) form. On the chembox front pbchemCID appears to be a copper(I) form despite errors in charge and has 5 hydrogen atoms; CompTox Dashboard (EPA) appears to just copy Wikipedia. ChecmspiderID is also a copper(I). CASNo is for a substance with 6 hydrogens. It references: https://pubs.acs.org/doi/abs/10.1021/ol102407k which is a Cu(II) and has formula with 4 hydrogen atoms and is a brown powder. This brown powder appears to be the same as the dehydrated form seen in https://link.springer.com/article/10.1007/BF02907224 . So hopefully @Smokefoot: you can determine what this article should be about. Does a disalicylate exist? Graeme Bartlett (talk) 23:03, 31 October 2023 (UTC)
- CSD has about 80 structures of adducts. Focusing on the Cu(O2CC6H4OH)2(H2O)x, there are a few examples, Then, focusing on Cu(O2CC6H4O)(H2O)x (phenoxide-carboxylate), I could find only one structure. A problem with Cu(II) is speciation in solution, so we dont have those data. In terms of Cu(I), I found one but its got PPh3 stabilizing it. Ullmann's has articles on salicylic acid (and derivs) and "copper compounds", neither article mention copper salicylate, so I conclude that the material is of little or no commercial value. Inorg Synthesis and Brauer's preparative compendium do not mention it. So one preliminary idea might be to make copper salicylate a listing of various hydrates of the Cu(II) material. --Smokefoot (talk) 23:30, 31 October 2023 (UTC)
- My inclination would be to delete this article as it refers to non-notable substance(s). We hardly need articles on all possible salts of a given parent, although some may be warranted, e.g. sodium salicylate. However, I've no objection if someone wants to put in the effort to salvage something. Mike Turnbull (talk) 10:53, 1 November 2023 (UTC)
- I have no problem wiping it out but if you try AFD, you will find that several nonchemical editors ("Elvis editors") suddenly appear and will offer their sage advice and deep concern that something precious is infringed. --Smokefoot (talk) 12:29, 1 November 2023 (UTC)
- I think a WP:TNT is warranted. Blow it up and start over. Graeme Bartlett (talk) 21:30, 1 November 2023 (UTC)
- We could also redirect to copper benzoate, which we could spruce up by listing various derivatives.--Smokefoot (talk) 02:30, 2 November 2023 (UTC)
- I think a WP:TNT is warranted. Blow it up and start over. Graeme Bartlett (talk) 21:30, 1 November 2023 (UTC)
- I have no problem wiping it out but if you try AFD, you will find that several nonchemical editors ("Elvis editors") suddenly appear and will offer their sage advice and deep concern that something precious is infringed. --Smokefoot (talk) 12:29, 1 November 2023 (UTC)
- My inclination would be to delete this article as it refers to non-notable substance(s). We hardly need articles on all possible salts of a given parent, although some may be warranted, e.g. sodium salicylate. However, I've no objection if someone wants to put in the effort to salvage something. Mike Turnbull (talk) 10:53, 1 November 2023 (UTC)
- CSD has about 80 structures of adducts. Focusing on the Cu(O2CC6H4OH)2(H2O)x, there are a few examples, Then, focusing on Cu(O2CC6H4O)(H2O)x (phenoxide-carboxylate), I could find only one structure. A problem with Cu(II) is speciation in solution, so we dont have those data. In terms of Cu(I), I found one but its got PPh3 stabilizing it. Ullmann's has articles on salicylic acid (and derivs) and "copper compounds", neither article mention copper salicylate, so I conclude that the material is of little or no commercial value. Inorg Synthesis and Brauer's preparative compendium do not mention it. So one preliminary idea might be to make copper salicylate a listing of various hydrates of the Cu(II) material. --Smokefoot (talk) 23:30, 31 October 2023 (UTC)
- In 2019 Edgar181 changed this from a disalicylate to the mono. In all the early history there was no reference. I added a referenced statement, but that was for a basic copper(II) form. On the chembox front pbchemCID appears to be a copper(I) form despite errors in charge and has 5 hydrogen atoms; CompTox Dashboard (EPA) appears to just copy Wikipedia. ChecmspiderID is also a copper(I). CASNo is for a substance with 6 hydrogens. It references: https://pubs.acs.org/doi/abs/10.1021/ol102407k which is a Cu(II) and has formula with 4 hydrogen atoms and is a brown powder. This brown powder appears to be the same as the dehydrated form seen in https://link.springer.com/article/10.1007/BF02907224 . So hopefully @Smokefoot: you can determine what this article should be about. Does a disalicylate exist? Graeme Bartlett (talk) 23:03, 31 October 2023 (UTC)
I noticed that the chemical structure shown in the article contains a symbol and curly brackets. I am not aware of any other polymer article with a similar structure. Unfortunately, MOS:CHEM (incl. subpages) does not provide guidance on how to draw chemical structures of polymers. I would propose removing { } from the structural formula. Any views on that? Leyo 12:06, 3 November 2023 (UTC)
- Polythene just uses a subscript n, which I think is pretty standard. would imply the whole universe was made of nothing but polymethylsiloxane, which wasn't true the last time I looked! Mike Turnbull (talk) 12:26, 3 November 2023 (UTC)
- P.S. The MOS does say
Formulae should be readily available, variables like n, x, or y are permissible for substances of variable composition such as polymers.
Mike Turnbull (talk) 12:39, 3 November 2023 (UTC)- What about the following changes:
- Removing
{ } - Adding
nnext to the closing square bracket - Should it still be
· n H2Oor rather a different variable (since the number of water molecule does not need to be the same as the number of monomers)?
- Removing
- --Leyo 13:32, 3 November 2023 (UTC)
- What about the following changes:
- P.S. The MOS does say
- You guys are focused on minutia of formating.... The article is unconvincing and reads like a sales pitch, also kinda teachy. This article might be removed, but it does serve a useful purpose in reminding readers that the former USSR was a source of low-level pseudoscientific crap. Some excerpts:
- "This product was synthesized in the late 1970s - early 1980s in the USSR by I. B. Slinyakova and I. М. Samodumova of L. B. Pisarzhevskiy Institute of Physical Chemistry[2] (Kyiv), where the theoretical foundations for the formation of the porous structure of organosilicon adsorbents with an adjustable porous structure and a pre-defined chemical nature of the surface were established; "
- just nonsense. How would "theory" inform anyone of this ordered double stranded siloxane vs myriad options? Silicates are notorious for crosslinking and complications.
- "The study of the use of the preparation was conducted in cooperation with the Medical Service of Krasnoznamennyi Kiev Military District (Medical Service Colonel Professor F. G. Novikov, Medical Service Colonel N. P. Bezlyuda)....The preparation is registered in Russia and the CIS countries ..." More shout outs. --Smokefoot (talk) 12:49, 3 November 2023 (UTC)
- I think Smokefoot is right, considering the significant contributions to the article by an account whose username matches the brand name of the product Enterosgel. Contributions from that user should be removed (or, at the very least, reviewed with a critical eye). Marbletan (talk) 13:18, 3 November 2023 (UTC)
- Yes, there are issues with the article (similar issues are currently also discussed in deWP). I just intended to resolve a (smaller) issue that may be fixed quickly. --Leyo 13:27, 3 November 2023 (UTC)
- I think Smokefoot is right, considering the significant contributions to the article by an account whose username matches the brand name of the product Enterosgel. Contributions from that user should be removed (or, at the very least, reviewed with a critical eye). Marbletan (talk) 13:18, 3 November 2023 (UTC)
The toxicity listing "coho salmon: LC50 = 95 ng/L" should be change to coho salmon: "LC50 = 0.095 micrograms/L" to be consistent with the units of other entries. Also, consider changing the mu symbol for micrograms to mcg as is used in the medical field as it is less likely to be confused with m = milli. Rgbutler (talk) 17:15, 8 November 2023 (UTC)
- All units now in μg/L. We use standard SI symbols. --Project Osprey (talk) 18:21, 8 November 2023 (UTC)
I'm not sure if the following sentence should really be in the article:
I was not able to find the (peer-reviewed) published version of that manuscript. --Leyo 13:26, 17 November 2023 (UTC)
- Agreed ChemRxiv is a preprint server, nothing there passes our usual standard (arXiv itself fails Wikipedia:Reliable sources/Perennial sources). I'm sure there are peer reviewed routes by now.--Project Osprey (talk) 23:06, 23 November 2023 (UTC)
- So far I haven't found any. doi:10.1126/science.abo5785 mentions a commercial standard that has become available. --Leyo 14:44, 24 November 2023 (UTC)
- Agreed ChemRxiv is a preprint server, nothing there passes our usual standard (arXiv itself fails Wikipedia:Reliable sources/Perennial sources). I'm sure there are peer reviewed routes by now.--Project Osprey (talk) 23:06, 23 November 2023 (UTC)
References
- ^ Agua, Alon; Stanton, Ryan; Pirrung, Michael (2021-02-04). "Preparation of 2-((4-Methylpentan-2-Yl)amino)-5-(Phenylamino)cyclohexa-2,5-Diene-1,4-Dione (6PPD-Quinone), an Environmental Hazard for Salmon" (PDF). ChemRxiv. doi:10.26434/chemrxiv.13698985.v1. S2CID 234062284.
Discussion at Wikipedia:Village pump (proposals)
![]() You are invited to join the discussion at Wikipedia:Village pump (proposals)#I have came up with new consistency in some articles of chemical compounds. Gain consensus from most other readers and helpful volunteers staying., which is within the scope of this WikiProject. DMacks (talk) 13:18, 29 November 2023 (UTC) DMacks (talk) 13:18, 29 November 2023 (UTC)
You are invited to join the discussion at Wikipedia:Village pump (proposals)#I have came up with new consistency in some articles of chemical compounds. Gain consensus from most other readers and helpful volunteers staying., which is within the scope of this WikiProject. DMacks (talk) 13:18, 29 November 2023 (UTC) DMacks (talk) 13:18, 29 November 2023 (UTC)
- Nothing at all. DMacks (talk) 01:12, 30 November 2023 (UTC)
 Resolved
Resolved
Creation of missing chemical articles - importance
It doesn't seem immediately relevant to the worklist, but there are a lot of chemicals relevant to lab and industry that do not have articles yet. I asked about this in the teahouse since I didn't know where to go with it and was directed to the red links in List of insecticides, List of herbicides, and List of fungicides as a less overwhelming project than the huge scope of User:Graeme_Bartlett/missing_chemicals and one that is more likely to have a broader range of secondary sources. The list of missing chemicals directed me to Bromopentane from Amyl bromide, which has questionable usefulness compared to just creating articles for the few bromopentanes that exist and makes me wonder how many of these pseudo-disambiguation pages exist for different kinds of substituted alkanes... What approach should I take in cleaning up the missing chemicals, especially the simplersubstituted hydrocarbons? Reconrabbit (talk) 18:55, 29 November 2023 (UTC)
- Indeed, "there are a lot of chemicals relevant to lab and industry that do not have articles yet". There are a also lot of chemicals relevant to lab and industry that do have articles. Where did these articles come from? The predicament is this: the project has grand scope, pretty high standards, but few regular editors. These editors must curate a hefty flux of edits. The same handful of editors then must choose between improving what we have or creating new articles. We are also constrained by "notability" and reliability. So my point is that if you are suggesting that the Chemistry project is imperfect and incomplete, we know but we are proud of what we've produced.
- A good way for you to start is to give us some detail of what is needed. Yours imperfectly, --Smokefoot (talk) 23:41, 29 November 2023 (UTC)
- I apologize - I'm very new to all of this and have been (and continue to be) massively impressed with the pages that are here and how far reaching the scope is. I am not a subject matter expert - I tend not to spend too much time in any particular lab in my profession - but I would like to contribute to the project with the tools available. Many of the articles in the worklist have since been improved significantly since they were first listed, and I'm just looking to be pointed somewhere to start, whether it's adding Chemdraw images to pages without them or filling out the red links on the list of inorganic compounds. Reconrabbit (talk) 01:19, 30 November 2023 (UTC)
- If you've just starting editing then I would suggest discreet small molecules to start with. They can be broken down into obvious sections (chembox, lead, synthesis, structure and properties, reactions etc) and cover all the key areas of editing. You could start from scratch or improve one of our stub articles - perhaps propyl gallate, that page is fairly basic but you've probably eaten some of it this week. I would stay clear of things like pesticides unless you have skills in understanding all of the toxicity issues, ditto medicines where we have very high standards for sources. We discourage people from citing their own work, but it is still good to start with what you know. Smokefoot is correct, our numbers are few, I would estimate the global pool of regular chem editors for the English language pages to be no more than 20. --Project Osprey (talk) 01:33, 30 November 2023 (UTC)
- There are several backlogged queues of requests for chemical diagrams. Someone with chemical knowledge and a copy of ChemDraw could make a bunch of contributions without having to dedicate a large amount of time to lit research or learning too many subtle details of WP writing. DMacks (talk) 19:22, 30 November 2023 (UTC)
- I recall seeing that list of requested drawings but haven't been able to find it again. Do you have a link? It might be more helpful to start on the drawings that don't exist yet over the ones that just need to have a .SVG format. I previously had academic access to ChemDraw but don't know if it's still valid - using BIOVIA Draw right now. Reconrabbit (talk) 19:35, 30 November 2023 (UTC)
- They are tracked in several different places:
- Category:Chembox articles without image
- Category:Infobox drug articles without a structure image (though many are proteins or other non-simple macromolecules)
- commons:Category:Low quality chemical diagrams and its /expired subcat (many of these are chemically deficient, not just looking for SVG to replace a high-quality PNG)
- commons:Category:Disputed chemical diagrams and its /expired subcat (substantive factual concerns)
- Wikipedia:WikiProject Chemistry/Image Request (no longer actively used)
- DMacks (talk) 19:48, 30 November 2023 (UTC)
- There are established guidelines for drawing chemicals, so in theory the drawing package shouldn't matter too much wikipedia:Manual of Style/Chemistry/Structure drawing--Project Osprey (talk) 22:14, 30 November 2023 (UTC)
- Definitely true. Some are better/easier than others to actually implement that MOS. I only mentioned CD because it's one of the ones that's better at it (at a ridiculous cost!), and what Reconrabbit specifically mentioned in their original post. DMacks (talk) 22:29, 30 November 2023 (UTC)
- @Reconrabbit I use BIOVIA Draw all the time, with Inkscape to convert its .emf image exports to .svg format. Ping me to your Talk Page if you have any issues doing the drawings. Note that our MOS linked by Project Osprey recommends using ACS document settings. My efforts are on Commons here. Mike Turnbull (talk) 11:20, 1 December 2023 (UTC)
- Definitely true. Some are better/easier than others to actually implement that MOS. I only mentioned CD because it's one of the ones that's better at it (at a ridiculous cost!), and what Reconrabbit specifically mentioned in their original post. DMacks (talk) 22:29, 30 November 2023 (UTC)
- There are established guidelines for drawing chemicals, so in theory the drawing package shouldn't matter too much wikipedia:Manual of Style/Chemistry/Structure drawing--Project Osprey (talk) 22:14, 30 November 2023 (UTC)
- They are tracked in several different places:
- I recall seeing that list of requested drawings but haven't been able to find it again. Do you have a link? It might be more helpful to start on the drawings that don't exist yet over the ones that just need to have a .SVG format. I previously had academic access to ChemDraw but don't know if it's still valid - using BIOVIA Draw right now. Reconrabbit (talk) 19:35, 30 November 2023 (UTC)
- There are several backlogged queues of requests for chemical diagrams. Someone with chemical knowledge and a copy of ChemDraw could make a bunch of contributions without having to dedicate a large amount of time to lit research or learning too many subtle details of WP writing. DMacks (talk) 19:22, 30 November 2023 (UTC)
- If you've just starting editing then I would suggest discreet small molecules to start with. They can be broken down into obvious sections (chembox, lead, synthesis, structure and properties, reactions etc) and cover all the key areas of editing. You could start from scratch or improve one of our stub articles - perhaps propyl gallate, that page is fairly basic but you've probably eaten some of it this week. I would stay clear of things like pesticides unless you have skills in understanding all of the toxicity issues, ditto medicines where we have very high standards for sources. We discourage people from citing their own work, but it is still good to start with what you know. Smokefoot is correct, our numbers are few, I would estimate the global pool of regular chem editors for the English language pages to be no more than 20. --Project Osprey (talk) 01:33, 30 November 2023 (UTC)
Category:Substances discovered in the 19th century
@1234qwer1234qwer4: Seems like questionable cat, in part because it will cover a lot of topics and in part because few really care. --Smokefoot (talk) 14:02, 25 December 2023 (UTC)
cover a lot of topics
can presumably be solved by subcategorisation, andfew really care
does not seem like a policy we have? What about the other categories like Category:Substances discovered in the 20th century etc., created in 2018 by @Leiem? 1234qwer1234qwer4 15:05, 25 December 2023 (UTC)- Seems a reasonable part of Category:Science by century. One could argue whether this is a defining characteristic of each substance. But it does seem like there is widespread (i.e., other fields of science) use of this overall tree, so I don't think chemistry should reject it on our own. If someone(s) care enough to maintain it, that's up to their use of their time. DMacks (talk) 05:42, 26 December 2023 (UTC)