Wikipedia talk:WikiProject Chemicals/Archive 2016
| This is an archive of past discussions on Wikipedia:WikiProject Chemicals. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 2010 | ← | Archive 2014 | Archive 2015 | Archive 2016 | Archive 2017 | Archive 2018 | → | Archive 2020 |
Image in nebivolol article

Help requested. I found the image File:Nebivolol_synthesis.svg (as seen at left) on the nebivolol page. The N atoms in structures 4 and 5 appear to me to be hanging in space way away from the rest of the structure. The uploader has been banned for violating rules on multiple accounts. Would someone please correct the images / upload new ones? Thanks. EdChem (talk) 08:43, 28 December 2015 (UTC)
- Changed (using Inkscape) Graeme Bartlett (talk) 11:22, 28 December 2015 (UTC)
- Thanks. :) EdChem (talk) 13:56, 28 December 2015 (UTC)
- Lots more weirdness...the alkene disappears and then reappears. And there is a butyl counterion for the trimethylsulfonium. And the caption for its use-case makes no sense. Yet again demonstrating why this editor's eits of this type should be removed as a default, and then simeone with time and interest can verify them. DMacks (talk) 05:20, 31 December 2015 (UTC)
- @DMacks: Do you mean my work, or the uploader of the image? I had noticed the alkene disappearing, which made sense under hydrogenating conditions, but I had not noticed it reappear. Perhaps the trimethylsulfonium cation is meant to be paired with bromide rather than butyl? In that case, the oxirane formation would occur by the Johnson–Corey–Chaykovsky reaction and the missing double bond should be re-added to structure 2. EdChem (talk) 05:47, 31 December 2015 (UTC)
- Sorry, I meant the banned uploader, whose contributions have been subject of several threads here. Your (EdChem and Graeme) edits are a great asset! Byt for the image to survive, it meed to be in accord with...whatever its cited published source is. I assume[original research?] the double bond should be gone due to hydrogenation and the never rturn (basedon the claimed target chemical identity) and that the counterion ahould be bromide or iodide. DMacks (talk) 05:58, 31 December 2015 (UTC)
- @DMacks: Do you mean my work, or the uploader of the image? I had noticed the alkene disappearing, which made sense under hydrogenating conditions, but I had not noticed it reappear. Perhaps the trimethylsulfonium cation is meant to be paired with bromide rather than butyl? In that case, the oxirane formation would occur by the Johnson–Corey–Chaykovsky reaction and the missing double bond should be re-added to structure 2. EdChem (talk) 05:47, 31 December 2015 (UTC)
- Lots more weirdness...the alkene disappears and then reappears. And there is a butyl counterion for the trimethylsulfonium. And the caption for its use-case makes no sense. Yet again demonstrating why this editor's eits of this type should be removed as a default, and then simeone with time and interest can verify them. DMacks (talk) 05:20, 31 December 2015 (UTC)
- Thanks. :) EdChem (talk) 13:56, 28 December 2015 (UTC)
Ok, according to this patent, the alkene functionality should not have returned, consistent with the final structure. The trimethylsulfonium's counter-ion is iodide. Also, the F on the benzene ring should be para to the ether linkage... EdChem (talk) 06:35, 31 December 2015 (UTC)
- So are the fixes required: 1 move all the -F links up one carbon on each ring; 2. Change Bu− to I−; and 3 change the double bond next to the caboxylic group to a single bond in steps 3 and above? Also the final product has the brige between the rings in the wrong carbon atom, whould be on the one next to the ether link. But is the initial product wrong too? I can probably do this in a few days time. Graeme Bartlett (talk) 23:03, 31 December 2015 (UTC)
- Thanks, Graeme, but I would hold off on the changes. The comment on the image in the article indicates the synthesis is not for nebivolol at all (hence the F isomers comment) and the patent I have linked to above seems to indicate a different starting material. It's odd, though, in that it suggests the carboxylic acid is reduced to a primary alcohol and then oxidized to an aldehyde, which strikes me as oddly inefficient. I'd like to put together a correct synthesis (I'll cut and paste images together) and then request someone draw it properly. Regards. EdChem (talk) 23:12, 31 December 2015 (UTC) PS: There are also stereochemical issues which the current image completely skips, it appears that the diastereomers of the oxirane are separated chromatographically, then one is reacted with the benzylamine, then the N-benzyl protected product formed by introducing the second. I think we are best off starting again for this image. EdChem (talk) 23:16, 31 December 2015 (UTC)
- That would explain the difference then. I am in no hurry! Though the article is not clear to me that the synthesis is not nebivolol. Probably we should remove the diagram from the article until it is correct. Graeme Bartlett (talk) 23:28, 31 December 2015 (UTC)
- I have commented out the whole section as the image is not the nebivolol synthesis and the description relates to the image. EdChem (talk) 06:35, 1 January 2016 (UTC)
- That would explain the difference then. I am in no hurry! Though the article is not clear to me that the synthesis is not nebivolol. Probably we should remove the diagram from the article until it is correct. Graeme Bartlett (talk) 23:28, 31 December 2015 (UTC)
- Thanks, Graeme, but I would hold off on the changes. The comment on the image in the article indicates the synthesis is not for nebivolol at all (hence the F isomers comment) and the patent I have linked to above seems to indicate a different starting material. It's odd, though, in that it suggests the carboxylic acid is reduced to a primary alcohol and then oxidized to an aldehyde, which strikes me as oddly inefficient. I'd like to put together a correct synthesis (I'll cut and paste images together) and then request someone draw it properly. Regards. EdChem (talk) 23:12, 31 December 2015 (UTC) PS: There are also stereochemical issues which the current image completely skips, it appears that the diastereomers of the oxirane are separated chromatographically, then one is reacted with the benzylamine, then the N-benzyl protected product formed by introducing the second. I think we are best off starting again for this image. EdChem (talk) 23:16, 31 December 2015 (UTC)
Shouldn't this article have a chembox or at least a maintenance category concerning this failure? --Leyo 01:36, 30 December 2015 (UTC)
- Some of the material is copied from https://www.thechemco.com/chemical/atbc-nature-flexx-509/. --Smokefoot (talk) 03:42, 30 December 2015 (UTC)
- I reverted the part that seemed to be plagiarism. I also moved the article to acetyltributylcitrate, but maybe that was unwise. On a more positive note, I did search this thing on SciFinder - a lot of references, so its the real deal although no applications reviews in English. --Smokefoot (talk) 03:49, 31 December 2015 (UTC)
- It mentioned in Regulators And Retailers Raise Pressure On Phthalates as a potential replacement of DEHP and DINP. --Leyo 20:56, 31 December 2015 (UTC)
- I reverted the part that seemed to be plagiarism. I also moved the article to acetyltributylcitrate, but maybe that was unwise. On a more positive note, I did search this thing on SciFinder - a lot of references, so its the real deal although no applications reviews in English. --Smokefoot (talk) 03:49, 31 December 2015 (UTC)
Structure diagram looks incorrect (per talkpage)...extra carbon in the linkage from the central carbon to one of the butylesters. Probably some other chembox data also would need to be updated to match that change. DMacks (talk) 06:42, 31 December 2015 (UTC)
- Slashme may be willing to fix his structure. --Leyo 20:56, 31 December 2015 (UTC)
- ...which he did. Thanks, User:Slashme! DMacks (talk) 23:02, 1 January 2016 (UTC)
- Always a pleasure ;-] --Slashme (talk) 23:18, 1 January 2016 (UTC)
- On a sidenote, de.wikipedia prefers structures like this, i.e. such a geometry but with explicit terminal CH3 groups. --Leyo 00:58, 2 January 2016 (UTC)
- OK, I understand the preference for a more natural geometry, but I'm surprised that they prefer the explicit terminal CH3 groups. That looks cluttered and confusing to me: it makes it harder to see the heteroatoms. But OK, consensus is consensus and I'm not a German chemist. Would you like me to draw a version like that? It would be quick and easy. --Slashme (talk) 13:14, 3 January 2016 (UTC)
- I also dont like the explicit CH3's with a stick drawing. Publishing chemists dont use that method. I recommend going one way or the other, totally stick or all explicit. For smallish molecules of interest to nonchemists, the more explicit, the better IMHO. --Smokefoot (talk) 14:35, 3 January 2016 (UTC)
- I invite editors to review the ASAPs at JOC (http://pubs.acs.org/toc/joceah/0/0) and ACIE (http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1521-3773/earlyview) to confirm that CH3 and stick drawings are not typically mixed together by professional chemists. --Smokefoot (talk) 15:29, 3 January 2016 (UTC)
- You cannot compare journal and Wikipedia articles 1:1. Wikipedia articles do not have a space limitation and are more frequently read by non-specialists. Hence, also the use of abbreviations such as “Me”, “Et”, “Pr” etc. is discouraged. --Leyo 16:39, 3 January 2016 (UTC)
- Oh, apologies, I didnt mean to advocate “Me”, “Et”, “Pr”. I am however asking that the community discuss the idea of not mixing CHn and stick diagrams. Use one or the other. Regarding the non-specialists, they would appreciate explicit drawings with CHn vertices. Professional chemists typically do not mix these two styles. --Smokefoot (talk) 17:07, 3 January 2016 (UTC)
- The advantage of explicit terminal groups (–CH3, =CH2, ≡CH) is that their meaning is always clear. The end of a line may also stand e.g. for a connection to a moiety (“R”). Anyhow, my comment above was just to inform that the preferences among different Wikipedias are not identical. --Leyo 20:00, 3 January 2016 (UTC)
- I definitely agree with Smokefoot that avoiding explicit terminal CH3 and other groups is desirable as the standard approach in major texts / references. EdChem (talk) 06:11, 8 January 2016 (UTC)
- The advantage of explicit terminal groups (–CH3, =CH2, ≡CH) is that their meaning is always clear. The end of a line may also stand e.g. for a connection to a moiety (“R”). Anyhow, my comment above was just to inform that the preferences among different Wikipedias are not identical. --Leyo 20:00, 3 January 2016 (UTC)
- Oh, apologies, I didnt mean to advocate “Me”, “Et”, “Pr”. I am however asking that the community discuss the idea of not mixing CHn and stick diagrams. Use one or the other. Regarding the non-specialists, they would appreciate explicit drawings with CHn vertices. Professional chemists typically do not mix these two styles. --Smokefoot (talk) 17:07, 3 January 2016 (UTC)
- You cannot compare journal and Wikipedia articles 1:1. Wikipedia articles do not have a space limitation and are more frequently read by non-specialists. Hence, also the use of abbreviations such as “Me”, “Et”, “Pr” etc. is discouraged. --Leyo 16:39, 3 January 2016 (UTC)
- I invite editors to review the ASAPs at JOC (http://pubs.acs.org/toc/joceah/0/0) and ACIE (http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1521-3773/earlyview) to confirm that CH3 and stick drawings are not typically mixed together by professional chemists. --Smokefoot (talk) 15:29, 3 January 2016 (UTC)
- I also dont like the explicit CH3's with a stick drawing. Publishing chemists dont use that method. I recommend going one way or the other, totally stick or all explicit. For smallish molecules of interest to nonchemists, the more explicit, the better IMHO. --Smokefoot (talk) 14:35, 3 January 2016 (UTC)
Amino acid articles introduction
Many articles of alpha-amino acids have very generic introductions. I've checked history and it looks like students in UCLA course were supposed to change them (see instructor's page with the template text). Changes removed useful information, internal links, references etc. and introduced generic text. (Text also contains improperly written formulae, for example "-+NH3".)
Some change diffs: alanine, methionine, tryptophan (all changes related to this course).
Should these changes be reverted? --Domen (talk) 23:08, 7 January 2016 (UTC)
The motivation behind the changes was to standardize the amino acid intros so that all the same important information was there. While the type of information is the same it is specific to the amino acid in question. In terms of informing new readers about amino acids we thought that would be a useful way to introduce the amino acids. I did post on the Wikiprojects chemistry talk page about the intended changes, I didn’t realize there was a separate one for chemicals. We didn’t intend for internal links and references to be removed. I will attempt to put those back as soon as I have a chance.
What additional information does anyone think should be included in the introduction?
The incorrect formula is my fault and I will correct those as well.
I would hope that you would not revert the intros as we found that some of them were confusing and had unnecessary information in them. Rather if you can improve on the changes we made we would greatly appreciate it. Htienson (talk) 00:29, 8 January 2016 (UTC)
- Most regular editors are watching both this and the Chemistry Project Talk page. I will say that student projects can be some of the most annoying that Wikipedia endures because of the lack of consultation, lack of knowledge, and the fact that they are generally led by instructors with no track record with Wikipedia who often leave us with advice on how regular editors should behave. --Smokefoot (talk) 01:56, 8 January 2016 (UTC)
- I think it is a shame that the standardised content was not discussed in advance. I think that not having a condensed structural formula in the lede of amino acid articles is a problem, and I wonder if the detailed discussion of ionic states and structural forms might not be better as a "Speciation" first section. The lede could say that XXX is typically represented by a condensed structural form NH2CH(R)COOH but that it has multiple ionic and zwitterionic forms. Classifying as aromatic / aliphatic and polar / non-polar side chains could be stated in the lede and explained also in speciation. Some of the new images with a blue side chain and different formats for charges is unfortunate. I also note the inconsistencies (in alanine, say) is named in a racemic form but illustrated stereospecifically - so is the article about L-alanine or DL-alanine or L-alanine and D-alanine. There is great opportunity to improve and standardise the amino acid articles, but I don't think it can be as simple as cut-and-paste fix with a couple of [pick correct option] inserts. I can see that this approach helps students to understand how amino acids are classified but I am much less sure that it helps our readers to an understanding of the topic. Student projects need to serve an educational function for the participants but also benefit the readers and it is unfortunate how often projects leave behind changes which need cleanup to best suit the readership. Htienson, that is part of the reason for Smokefoot's irritation as students learning chemistry have repeatedly been set to changing articles on topics they are only starting to understand and where some content is ahead of their comprehension, creating work for volunteers. As an instructor, I might set my students a task for me to assess explaining content, but I would not use those writings as authoritative sources for others under most circumstances. Might I suggest that a discussion of future projects to include Wikipedian content editors' perspectives would be desirable? EdChem (talk) 06:07, 8 January 2016 (UTC)
- From a biochemistry perspective we felt the representation of the amino acids in the non-ionic form was particularly unfortunate, as it is physiologically irrelevant given its miniscule concentration. Our main focus was to have the amino acids represented in their most physiologically relevant form, as they are biological molecules. In asking about this it was mentioned that the ionic forms should be explained in the introduction, as this may confuse readers who weren’t as familiar with pKas. We thought this might be a good opportunity to standardize the introductions and decided together what content we thought would be most useful to students learning about amino acids. As they are students I think their perspective on this topic was particularly useful. Given that we were coming more from a biochemical/biological perspective we didn’t think the condensed structure was necessary in the introduction.
- We liked the idea of the side chain in a different color to distinguish the unique part of that particular amino acid relative to others.
- As I mentioned before I have no problem cleaning up the issues that others have brought up when I have a chance. And as I am learning as well I will try to get more input from content editors along the way. Htienson (talk) 06:54, 8 January 2016 (UTC)
Aspartame images
I might be wrong, but to me the images in Aspartame are not identical (+ checking JMol 3D). The ball-and-stick seems to have the N-atoms (blue) different and incorrect. Shows an NH3. -DePiep (talk) 21:35, 20 January 2016 (UTC)
- By "different", do you mean the aspartic acid (neutral –CO2H and neutral –NH2) is instead in rhe zwitterionic form (CO2– and NH3+ respectively)? Or something else? The zwitterion is WP:V for the crystalline form. But commons also has File:Aspartame-3D-balls-2.png that is the same geometry but editted to be the neutral acid/amine form. DMacks (talk) 22:14, 20 January 2016 (UTC)
- I really don't know. I'm just counting balls by color, and their chain. One image has NH3, the other has NH2. Is the reader supposed to know that these are 'the same' somehow? -DePiep (talk) 22:27, 20 January 2016 (UTC)
- Does the C with the two O on the left also have a difference of a lack of an H instead? WP:CHEM really needs to write down the standard way (neutral functional groups vs "real world" zwitterionic, and vs physiological pH for biological entities and/or with counterions). I think you found yet another good example of the confusion we have otherwise. Is the other image I mentioned more consistent with the other structures you are seeing? DMacks (talk) 22:31, 20 January 2016 (UTC)
- "Does the C ..." - I don't know. I just compare the images. They don't say ions are involved. -DePiep (talk) 22:34, 20 January 2016 (UTC)
- re the other image you linked: well, counting the balls & atoms, it looks better. -DePiep (talk) 22:38, 20 January 2016 (UTC)
- Not to be rude, but the point is not to explain it to me, here now. Our Reader should get it. -DePiep (talk) 22:52, 20 January 2016 (UTC)
- I can think of about aleph_0 ways an image can be confusing to a reader in "some way" who is unversed to "some level" in "some piece of background" even if chemically correct. Remember, the claim was that "it's wrong in [a certain way]", and if the goal is to fix it, we need to know if there any other details that are not clear/correct/etc. It would be easy for someone to say "N has too many H, edit one out", and then it's not even chemically correct. Instead, if we know that specific way(s) you see when you know to look for them, we can make sure we're actually making it better not just different. As you have been interested in infoboxes in general and understand some chemistry, I would have assumed you'd also care about a general concern of consistency therein not just fixing this one case (otherwise why would you do anything except take the solution I offered in the very first response and just insert it?). DMacks (talk) 03:25, 21 January 2016 (UTC)
- Does the C with the two O on the left also have a difference of a lack of an H instead? WP:CHEM really needs to write down the standard way (neutral functional groups vs "real world" zwitterionic, and vs physiological pH for biological entities and/or with counterions). I think you found yet another good example of the confusion we have otherwise. Is the other image I mentioned more consistent with the other structures you are seeing? DMacks (talk) 22:31, 20 January 2016 (UTC)
- I really don't know. I'm just counting balls by color, and their chain. One image has NH3, the other has NH2. Is the reader supposed to know that these are 'the same' somehow? -DePiep (talk) 22:27, 20 January 2016 (UTC)
- The two images are indeed different. The first is a "canonical" version showing an amino group (NH2) and a carboxylic acid (CO2H). The second was the zwitterionic form showing an ammonium (NH3+) and a carboxylate (CO2-). Whether one or the other is more correct depends on context. But I think it is best to be consistent, so I switched the second to the image that DMacks notes above, File:Aspartame-3D-balls-2.png, which is consistent with the first image. Discussion of the relevance of a zwitterionic form can occur in the text if necessary. -- Ed (Edgar181) 13:45, 21 January 2016 (UTC)
Newbie
Dear Wikipedian colleagues, I've been around for some time, but I am still quite new to creating new pages. Now I have some free time and a copy of the Merck Index and CRC Handbook at hands. I propose myself to create new Wiki pages for less common inorganic compounds listed on those references. I ask you opinion if that is welcome and, if so, if you have any suggestions or recommendations for me at this time. Thanks! JEFCG (talk) 08:08, 28 January 2016 (UTC)
- Hello and welcome. You may find some inspiration at Wikipedia:Requested_articles/Natural_sciences/Chemistry. --Project Osprey (talk) 08:45, 28 January 2016 (UTC)
- Welcome indeed - thank you for your kind offer of help! I've noticed that many of the lanthanides only have articles on an oxide and a chloride (I wrote several of the latter), but few other salts. See Category:Neodymium_compounds for an example - amazing, when you think that neodymium is more common than tin or lead in the Earth's crust! If you fancy writing any, I'd be happy to look over what you've written; I may be able to provide pictures for one or two. Let me know! Thanks, Walkerma (talk) 01:23, 1 February 2016 (UTC)
Pages without CAS Registry Numbers
Category:Chemical pages without CAS Registry Number has been deleted under the criteria of WP:G8. I occasionally used this cat as a to-do-list of pages to improve and I would like it back (providing that its not a problem). I can't undo page deletions so can someone resort it for me?.. Also, can I suggest the use of the {{G8-exempt}} tag? --Project Osprey (talk) 10:00, 3 February 2016 (UTC)
- It has been renamed into Category:Chemical articles without CAS Registry Number (articles only). FYI, the CAS RN maintenance categories are listed in Category:CAS Registry Number maintenance categories. -DePiep (talk) 11:27, 3 February 2016 (UTC)
Does anyone know the structure of “extra-chlorine DDT or Cl-DDT” (see e.g. cornell.edu)? --Leyo 23:44, 12 February 2016 (UTC)
- Ullmann's Encyclopedia: nothing about an "extra chlorine" or Cl-DDTanything. Quoting about impurities in DDT "Technical DDT is a white amorphous powder containing 65 – 80% of the active 4,4-DDT; 14 – 21% of the nearly inactive 2,4-DDT [789-02-6], mp 73 – 74 ◦C; up to 4% of the insecticidal DDD [72-54-8], 1,1-(2,2-dichloroethylidene)bis(4 chlorobenzene), mp 110 ◦C; and traces of 2,2-DDT and bis(4-chlorophenyl)sulfone."
- On ChemAbs, nothing relevant for "extra-chlorine DDT" but "Cl-DDT" does give a few hits. It looks like Cl-DDT is a term used always with prefixes like p,p'- and o,p'- when one is discussing the specific isomers of the ClC6H4 substituents on DDT ("official DDT" is p,p'- isomer).
- A quote from one of the few hits that suggests that some people are interested in getting dicofol with minimal contamination of DDT: "Environmental Science and Pollution Research (2009), 16(2), 214-217. "....Since dicofol is mainly synthesized from dichlorodiphenyltrichlorethane (DDT), it contains DDT as an impurity. The European Community has forced Prohibition Directive 79/117/EEC to reduce DDT in dicofol formulations."--Smokefoot (talk) 04:58, 13 February 2016 (UTC)
- Thank you for digging into the literature. Would you be in favor of removing
and a substance called extra-chlorine DDT or Cl-DDTfrom Dicofol#Impurities, then? --Leyo 01:21, 14 February 2016 (UTC)
- Thank you for digging into the literature. Would you be in favor of removing
Category:Pages where template include size is exceeded
Hello. At Category:Pages where template include size is exceeded we are trying to empty the place, and it happens that this 13 pages
Wikipedia:WikiProject Chemicals/Log/2010-11-04
Wikipedia:WikiProject Chemicals/Log/2010-11-06
Wikipedia:WikiProject Chemicals/Log/2010-11-12
Wikipedia:WikiProject Chemicals/Log/2010-11-28
Wikipedia:WikiProject Chemicals/Log/2010-11-30
Wikipedia:WikiProject Chemicals/Log/2010-12-01
Wikipedia:WikiProject Chemicals/Log/2010-12-02
Wikipedia:WikiProject Chemicals/Log/2010-12-03
Wikipedia:WikiProject Chemicals/Log/2010-12-06
Wikipedia:WikiProject Chemicals/Log/2010-12-07
Wikipedia:WikiProject Chemicals/Log/2010-12-11
Wikipedia:WikiProject Chemicals/Log/2010-12-12
Wikipedia:WikiProject Chemicals/Log/2010-12-16
Wikipedia:WikiProject Chemicals/Log/2011-08-06
Wikipedia:WikiProject Chemicals/Log/2011-08-09
Wikipedia:WikiProject Chemicals/Log/2011-08-10
all created by CheMoBot, are on overflow. Perhaps, you could split each of them in 2 pages ? Pldx1 (talk) 22:33, 20 February 2016 (UTC)
- Are created and maintained by User:CheMoBot by Beetstra. A manual split would probably break bot control. Note: I am pondering to stop this bot process completely (by Talk). Do these pages disturb anything? -DePiep (talk) 22:51, 20 February 2016 (UTC)
- @DePiep and Pldx1: No, these pages do not disturb anything except for some who feel the urge to have that category empty (as they feel it obscures their abilitites to find the pages where exceeding a transclusion limit is a problem).
- Anywayz, these logs can plainly be deleted, no need for them at all anymore. --Dirk Beetstra T C 03:16, 21 February 2016 (UTC)
- Beetstra Some more from that same category:
Wikipedia:WikiProject Pharmacology/Log/2010-12-03
Wikipedia:WikiProject Pharmacology/Log/2011-06-30
Wikipedia:WikiProject Pharmacology/Log/2011-07-01
Wikipedia:WikiProject Pharmacology/Log/2011-08-10
Wikipedia:WikiProject Pharmacology/Log/2011-09-03
- -DePiep (talk) 21:28, 21 February 2016 (UTC):T
- There is an enormous amount of these. See here and here. Its a job for a bot or Special:Nuke. Christian75 (talk) 09:17, 22 February 2016 (UTC)
- They are not in the Size-exceeded-category this post is about, is it? -DePiep (talk) 10:15, 22 February 2016 (UTC)
- But if we delete some, we should delete them all. Which means its a part of the discussion to delete these pages you and other listed. Christian75 (talk) 10:33, 22 February 2016 (UTC)
- They are not in the Size-exceeded-category this post is about, is it? -DePiep (talk) 10:15, 22 February 2016 (UTC)
- There is an enormous amount of these. See here and here. Its a job for a bot or Special:Nuke. Christian75 (talk) 09:17, 22 February 2016 (UTC)
Discussion about generally considering articles from predatory publishers unreliable
There is a discussion here if that topic is of interest. It has been going on since Feb 26, but just wanted to make sure folks here are aware of it. Jytdog (talk) 18:04, 4 March 2016 (UTC)
Jmol interactive 3D structure of rhodizonic acid
Something seems to be wrong with the Jmol interactive 3D structure of rhodizonic acid. --Leyo 21:17, 18 March 2016 (UTC)
- Fixed. The Jmol structure is generated from the SMILES field of the infobox, so any mistake in the Jmol is either a mistake in the SMILES itself or in the machine conversion from that 2D structural description to a 3D molecular form. In this case, as the Jmol displays, there was a single bond that should have been double. Also, the carbons should have been capital "C" not lower-case "c" because they are not aromatic. DMacks (talk) 21:45, 18 March 2016 (UTC)
Problem with CAS number 152923-56-3
According to ChemIDplus, CAS number 152923-56-3 corresponds to Daclizumab (see here). But this CAS number is validated in the chembox of Basiliximab. Please contact the responsible of the validation bot to see if there is a modification to do in its reference list. Thank you Snipre (talk) 15:45, 19 March 2016 (UTC)
- The correct CAS# for basiliximab is 179045-86-4. I have corrected the data at the article, but I'm not sure how to change the validation. -- Ed (Edgar181) 16:55, 19 March 2016 (UTC)
About some proposals on WT:VA/E
I've proposed to add chlorofluorocarbon and Magnesium chloride to WP:VA/E, but they have been having no votes for at least 7 days. Hope that you can participate in the voting of these proposals. Thanks!--RekishiEJ (talk) 06:54, 20 March 2016 (UTC)
CAS number problem with Alglucerase and Taliglucerase alfa
Both articles have the same CAS number but according to ChemIDplus they have a different one:
- Alglucerase: 143003-46-7
- Taliglucerase alfa: 37228-64-1
Can someone provide another source for both compounds in order to validate these numbers ? Thank you Snipre (talk) 12:35, 22 March 2016 (UTC)
- SciFinder calls 143003-46-7 "Ceramidase, glucosyl- (human placenta isoenzyme protein moiety reduced)", a 497-residue protein, and 37228-64-1 "Glucosylceramidase". Having trouble getting a stable enough network connection to cross-check the refs for each. DMacks (talk) 14:09, 22 March 2016 (UTC)
Please check Monoiodotyrosine and 3-Iodotyrosine
From my understanding, Monoiodotyrosine is about one stereoisomer and 3-Iodotyrosine about the mixture of stereoiomers. Can someone check if this is true and change the appropriate picture ? Thank you. Snipre (talk) 14:42, 25 March 2016 (UTC)
- That appears to be the distinction based on PubChem IDs, but they have the same ChemSpider ID in their infobox. The PubChem entry for the mixture also includes just the L isomer as synonym, and spot-checking the journal articles listed there I'm only seeing the L being discussed. Even our one cited ref for the mixture's article is actually specifically about the L isomer. I could envision killing the mixture's article unless there's any separate notability of that. But I would think that "3-Iodotyrosine" is a better article title, so would have to reverse-merge or merge-and-rename/overwrite. Maybe putting the content in a new "3-iodo-L-tyrosine" (even more correct for that actual subject) and redirecting both to it, with edit-summary link to this here discussion would avoid making it any more confusing. DMacks (talk) 20:45, 30 March 2016 (UTC)
Please check and merge Polystyrene sulfonate and Tolevamer
@Anypodetos: From my understanding, these articles are about the same molecule. Can someone check if this is true and merge both articles ? Thank you. Snipre (talk) 12:23, 22 March 2016 (UTC)
- Now merged by User:Anypodetos. DMacks (talk) 02:05, 31 March 2016 (UTC)
Please check and merge Aureolin and Potassium cobaltinitrite
From my understanding, these articles are about the same molecule. Can someone check if this is true and merge both articles ? Thank you. Snipre (talk) 15:38, 19 March 2016 (UTC)
I have checked both articles and it is clear that they should be merged. It is indeed the same substance. I have never done any merging operations. So either you could merge the articles or point me to the instructions how to do it. Jurajlip 17:45, 23 March 2016 (UTC) — Preceding unsigned comment added by Jurajlip (talk • contribs)
- @Jurajlip: Thank you for your proposition to do it. Here is the explanation: Wikipedia:Merging#How_to_merge. I prefer that an author is doing this action because I don't want to modify a topic I don't know. Snipre (talk) 19:40, 30 March 2016 (UTC)
- I can do the merge, but am uncertain which direction to go (which title should be the actual article, vs the other as a redirect to it). The chemical-name is probably a more proper name of this substance itself but this article is newer; the pigment article is older and probably the most/only notable aspect of the substance, but it's only one aspect. I'll go with chemical-name as actual article tomorrow unless someone objects by then... DMacks (talk) 20:22, 30 March 2016 (UTC)
- @DMacks: Thank you. Snipre (talk) 10:26, 2 April 2016 (UTC)
- I can do the merge, but am uncertain which direction to go (which title should be the actual article, vs the other as a redirect to it). The chemical-name is probably a more proper name of this substance itself but this article is newer; the pigment article is older and probably the most/only notable aspect of the substance, but it's only one aspect. I'll go with chemical-name as actual article tomorrow unless someone objects by then... DMacks (talk) 20:22, 30 March 2016 (UTC)
Inconsistency in Zineb
In the article, it says “Zineb and is a polymeric complex of zinc …”. The structure shown, however, is a salt. Is there a better representation of the structure? --Leyo 20:17, 4 April 2016 (UTC)
- This page (Pesticide Properties DataBase, University of Hertfordshire) shows it as a polymer involving Zn-S covalent bonds (click "yes" next to "Structure diagram/image available?"). I'm not sure whether that is more accurate than the salt form currently shown in the article, but it is a reasonable depiction of "a polymeric complex of zinc". -- Ed (Edgar181) 20:24, 4 April 2016 (UTC)
- Thanks. What about showing both, then? --Leyo 07:55, 5 April 2016 (UTC)
- I would guess that the reality lies somewhere between the ionic and the covalent representations, but I don't know. Maybe one of them is just wrong and shouldn't be shown at all. -- Ed (Edgar181) 14:06, 5 April 2016 (UTC)
- Axiosaurus who created the article is currently inactive, but Stone who added the structure is still active. He may be able to help. --Leyo 15:21, 5 April 2016 (UTC)
- Does anyone have more insight into this? --Leyo 12:02, 13 April 2016 (UTC)
- There does seem to be an odd lack of XRD data. I've found a somewhat obscure journal which may contain a structure but I don't have access to it (doi:10.1135/cccc19801495). Anyone watching this got access? --Project Osprey (talk) 09:24, 14 April 2016 (UTC)
- Oh, let me check, its my area. Lots of Zn(II) dithiocarbamates, usually pentacoordinate. For some of these polymers, a wrong or highly simple ionic depiction can be more helpful to average reader than the Xray structure. But let me dig around. --Smokefoot (talk) 14:05, 14 April 2016 (UTC)
- There does seem to be an odd lack of XRD data. I've found a somewhat obscure journal which may contain a structure but I don't have access to it (doi:10.1135/cccc19801495). Anyone watching this got access? --Project Osprey (talk) 09:24, 14 April 2016 (UTC)
- I would guess that the reality lies somewhere between the ionic and the covalent representations, but I don't know. Maybe one of them is just wrong and shouldn't be shown at all. -- Ed (Edgar181) 14:06, 5 April 2016 (UTC)
- Thanks. What about showing both, then? --Leyo 07:55, 5 April 2016 (UTC)
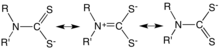
- Ok, I describe the structure in a more contemporary way. Slightly over simplified. Am waiting to see if someone has a crystallographic file on this thing. Nice to have a better structural part of the article, but 99% of readers are more interested in the applications and mech of action. --Smokefoot (talk) 22:23, 14 April 2016 (UTC)
- Thanks a lot for digging and providing an accurate structure.
- BTW: Propineb that doesn't have an en.wikipedia article yet (but de and fr) may be a similar case concerning having an oversimplified structure. --Leyo 09:34, 15 April 2016 (UTC)
- Ok, I describe the structure in a more contemporary way. Slightly over simplified. Am waiting to see if someone has a crystallographic file on this thing. Nice to have a better structural part of the article, but 99% of readers are more interested in the applications and mech of action. --Smokefoot (talk) 22:23, 14 April 2016 (UTC)
Re the new image, I know that the carbon-sulfur bonds are identical and neither single nor double but I wonder if the appearance of apparently trivalent carbons in the dithiocarbamate groups will be confusing? EdChem (talk) 13:10, 15 April 2016 (UTC)
- OK, let me do something. The main double-bonding is C=N and then one needs to write little charges everywhere etc. But thanks and will revise to avoid 3-bonded C. Thanks, --Smokefoot (talk) 13:14, 15 April 2016 (UTC)
- Having some troubles, the supposed Xray report is in a Bulgarian journal that will take some time to get. Also having trouble with some Xray databases. And if anyone's interested, above is an image inserted into dithiocarbamate, to show why I was struggling.--Smokefoot (talk) 00:57, 17 April 2016 (UTC)
There's nothing in the CSD. Just the sodium salt of the ligand and a cyclic tin complex. --Ben (talk) 09:10, 19 April 2016 (UTC)
- Thanks for checking. Are you referring to zineb or also to propineb? --Leyo 09:47, 19 April 2016 (UTC)
Nothing for propineb either, nor any zinc compounds with similar ligands (with an ethylene backbone linking two dithiocarbamate groups). There are about 250 structures containing some kind of dithiocarbamate and zinc. Many of them have zinc coordinated to four sulfurs and one nitrogen-donor ligand such as pyridine. There are also dimers and six-coordinate Zn, as well as a few of the tetrahedral (R2NCS2)2Zn species I expected to see. Perhaps polymers like zineb and propineb are too insoluble to crystallize. --Ben (talk) 16:32, 19 April 2016 (UTC)
Question
In general, if a compound is chiral, is its chirality a "noteworthy feature" (as described in MOS:CHEM/Chemicals#Introductory paragraph) that should be mentioned in the lead of an article on that compound? If it's not, it would help to get some input at Talk:MDMA/Archive 6#Chirality and drug classes about what chemistry-related content should be mentioned in that particular article, since there's currently no coverage of any of that drug's chemical properties in the lead. Seppi333 (Insert 2¢) 21:06, 26 April 2016 (UTC)
Need info about Rivanicline
Rivanicline is defined as (E)-metanicotine with CAS number 15585-43-0 on ChemIDplus and metanicotine is defined with CAS number 538-79-4 on ChemIDplus. But both ChemIDplus pages have the same sturcture (and same InChIKey).
Are both CAS numbers representing the same compound or CAS number 15585-43-0 represents the (E)-metanicotine and CAS number 538-79-4 the mixture of (E)-metanicotine and (Z)-metanicotine. Thank you in advance for your help. Snipre (talk) 21:24, 13 April 2016 (UTC)
Same problem with 252260-02-9 and 252260-06-3
Can someone checks if the compounds defined by 252260-02-9 and 252260-06-3 are the same or if there is a difference ? Thanks. Snipre (talk) 22:41, 13 April 2016 (UTC)
Same problem with 102-61-4 and 22139-77-1
Can someone checks if the compounds defined by 102-61-4 and 22139-77-1 are the same or if there is a difference ? Thanks. Snipre (talk) 22:54, 13 April 2016 (UTC)
- 15585-43-0 = (E)-metanicotine
- 538-79-4 = metanicotine (i.e. no double bond geometry assigned - is that still a racemate?)
- 252260-02-9 = Posizolid (2S, 5R)
- 252260-06-3 = Same base structure but the primary alcohol has been reacted to give a phosphate ester (a prodrug perhaps? It has a development name of AZD 2563DSP)
- 102-61-4 = Pinosylvin (no double bond geometry assigned)
- 22139-77-1 = (E)-Pinosylvin
- I've made basic changes to the pages to try and clarify this, if you want to look into the prodrug there's currently 1 paper at (doi:10.1021/op060003h) but after that you're down to patents. --Project Osprey (talk) 09:00, 14 April 2016 (UTC)
- I've added the magnus tool external links~by CAS number. Could help? -DePiep (talk) 18:13, 18 April 2016 (UTC)
- @DePiep: Thanks but most of the time, when dealing with stereoisomers, few databases provide good data. Snipre (talk) 05:41, 27 April 2016 (UTC)
- @DePiep and Project Osprey: Do you know or anyone else how can we contact ChemIDplus database ? Because for Posizolid ChemIDplus proposes the same molecule for both CAS numbers (see here and there). Drug Bank database proposes CAS number 252260-06-3 for Posizolid (see here). I miss a reference database to be able to show the difference between these two CAS numbers so if you want you can contact both databases to indicate the problem. Snipre (talk) 20:22, 28 April 2016 (UTC)
- In case here is the form to contact Drug Bank. They are quite open to modify their data if we can provide a source. Snipre (talk) 20:27, 28 April 2016 (UTC)
- Problems for this aren't unusual for compounds that have very little literature behind them (such as Posizoid), they usually get sorted out eventually. --Project Osprey (talk) 21:04, 28 April 2016 (UTC)
- @DePiep and Project Osprey: Do you know or anyone else how can we contact ChemIDplus database ? Because for Posizolid ChemIDplus proposes the same molecule for both CAS numbers (see here and there). Drug Bank database proposes CAS number 252260-06-3 for Posizolid (see here). I miss a reference database to be able to show the difference between these two CAS numbers so if you want you can contact both databases to indicate the problem. Snipre (talk) 20:22, 28 April 2016 (UTC)
- @DePiep: Thanks but most of the time, when dealing with stereoisomers, few databases provide good data. Snipre (talk) 05:41, 27 April 2016 (UTC)
- I've added the magnus tool external links~by CAS number. Could help? -DePiep (talk) 18:13, 18 April 2016 (UTC)
SMILES and Jmol
Two questions. -DePiep (talk) 11:30, 21 April 2016 (UTC)
Jmol different from SMILES
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
By default, {{Chembox}} uses the |SMILES= input to create a link to the external Jmol interactive image. That is: Jmol uses the SMILES linear definition. All fine, most of the time.
Some months ago I read in a talkpage that there are situations where Jmol should be build from a different SMILES string. I can't find that any more, so my question is: does anyone have an example for this situation?
I plan to build the option into {{Chembox}} to overwrite |SMILES= with new parameter |Jmol= to cover this situation. Note: also, entering |Jmol=none will suppress any Jmol data showing up. -DePiep (talk) 11:30, 21 April 2016 (UTC)
- I've mentioned that limitation a few times. The most common case is things like ferrocene. I don't know if I can write a SMILES that contains a covalent bond from each C to the central Fe in order to (probably) convince Jmol to render the thing visually correctly with respect to the ligand–metal associations. But that's not what the standard structure->SMILES generators seem to do. And it's not really correct anyway in terms of electronic structure, nor visually in terms of the C geometry. The generated one at least gets the rings correct, but totally breaks the arrangement of the ligands vs metal. DMacks (talk) 03:11, 26 April 2016 (UTC)
- Might be harder than I thought to even get it to look correct. I usually use MOLfile, XYZ, or similar formats for this sort of thing. I know Jmol can read those formats, but I don't know if what we embed in the infobox can be fed that data. DMacks (talk) 03:39, 26 April 2016 (UTC)
- Dimethyldiborane demonstrates another limitation: it doesn't know that the B should be tetrahedral. Probably more a bug in the external rendering applet with the 2D->3D conversion, but great example why we need local control when we know of external problems with the SMILES handling. DMacks (talk) 22:00, 28 April 2016 (UTC)
SMILES uppercase/lowercase diifference
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
Q2, possibly related: sometimes I see two SMILES for one substance, one having uppercases the other one having lowercases. Is this relevant for the Jmol image? IOW, do they both result in a correct Jmol image? (example needed added) -DePiep (talk) 11:12, 21 April 2016 (UTC)
- The lc example is broken. Is part of the answer? -DePiep (talk) 11:30, 21 April 2016 (UTC)
Upper and lower case 'c' code for normal and aromatic carbons respectively (see below). In drugbox you mostly see SMILES for aromatic compounds written with 'c' - but in chembox most use 'C'. It's possible to have a long debate about which representation we should be showing. Personally, I've noticed that many computer interfaces don't understand SMILES containing 'c' (Scifinder for example) and as SMILES is supposed to be machine readable I think that's a big problem. It's possible that this relates to the Jmol issue (why else would you need a second SMILES unless the first one doesn't work?) but I can't say so for certain. --Project Osprey (talk) 12:45, 21 April 2016 (UTC)
-
C1=CC=CC=C1
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
- OK, so they do represent a different image. For now, this is not about which SMILES form we should have in the box (to keep this simple).
- I'd like to learn whether we can blindly link to both SMILES forms, or that I can automate like "do not link to Jmol when lowercases in SMILES". At least tthat could prevent showing the systematically broken Nitroglycerin Jmol (2nd link). btw, I plan to copy this whole Jmol stuff into {{Drugbox}}, once stable. -DePiep (talk) 13:21, 21 April 2016 (UTC)
- Check: the lowercase nitroglycerine is broken for Jmol. The benzol SMILESs show the same image :-) . -DePiep (talk) 13:24, 21 April 2016 (UTC)
- Frustratingly Jmol seems to be able to handle some things but not others. The lowercase SMILES for nitroglycerin ( o:n(:o)OCC(COn(:o):o)On(:o):o ) is trying to represent the resonance hybrid of the nitro groups and that seems to be too much. Lower case c isn't a problem though. For a start I think strings containing o:n should be ignored - there may be more rules to come. --Project Osprey (talk) 14:33, 21 April 2016 (UTC)
- So. For me, coding {{Chembox}}, there is nothing to automate. We'll have the occasional Jmol misfit as with the -- SMILESwise correct -- nitroglycerin demo. In that case, one can add
|Jmol1=noneto suppress (hide) that faulty Jmol link. Will do. -DePiep (talk) 21:34, 21 April 2016 (UTC)- Yes, that seems like the easiest solution --Project Osprey (talk) 22:30, 21 April 2016 (UTC)
- I don't know if SMILES supports resonance in general, as opposed to aromaticity via lower-case letters. our SMILES article doesn't mention it. But it also mentions that ":" for aromatic bonds is optional. I just tried on(o)OCC(COn(o)o)On(o)o and got a decent-looking nitroglycerine with visual pi-bonding among the ONO components. I don't know if there are any differences internally between a normal double-bond and an aromatic/resonance-hybrid. For example, whether my result exceeds octet on N. DMacks (talk) 03:19, 26 April 2016 (UTC)
- So. For me, coding {{Chembox}}, there is nothing to automate. We'll have the occasional Jmol misfit as with the -- SMILESwise correct -- nitroglycerin demo. In that case, one can add
- Frustratingly Jmol seems to be able to handle some things but not others. The lowercase SMILES for nitroglycerin ( o:n(:o)OCC(COn(:o):o)On(:o):o ) is trying to represent the resonance hybrid of the nitro groups and that seems to be too much. Lower case c isn't a problem though. For a start I think strings containing o:n should be ignored - there may be more rules to come. --Project Osprey (talk) 14:33, 21 April 2016 (UTC)
- Check: the lowercase nitroglycerine is broken for Jmol. The benzol SMILESs show the same image :-) . -DePiep (talk) 13:24, 21 April 2016 (UTC)
There is a list of all Chemboxes with SMILES errors here (chose the 'Browse errors' option at the top). --Project Osprey (talk) 20:58, 28 April 2016 (UTC)
Still some problems with CAS numbers
Hi, I still have some problems to identify the correct structure from a CAS number:
- Case 1:
- Case 2:
- Case 3:
Thank you for your help. Snipre (talk) 14:44, 29 April 2016 (UTC)
MDMA RFC: Chemicals MOS and undue weight
Please contribute to the RFC on the MDMA talk page. Part of it concerns the application of the chemicals MOS to drug articles. Talk:MDMA#Chirality and drug class in lead Sizeofint (talk) 23:07, 29 April 2016 (UTC)
Substub since 2007.Xx236 (talk) 10:10, 30 May 2016 (UTC)
Auto-assessment of article classes
Following a recent discussion at WP:VPR, there is consensus for an opt-in bot task that automatically assesses the class of articles based on classes listed for other project templates on the same page. In other words, if WikiProject A has evaluated an article to be C-class and WikiProject B hasn't evaluated the article at all, such a bot task would automatically evaluate the article as C-class for WikiProject B.
If you think auto-assessment might benefit this project, consider discussing it with other members here. For more information or to request an auto-assessment run, please visit User:BU RoBOT/autoassess. This is a one-time message to alert projects with over 1,000 unassessed articles to this possibility. ~ RobTalk 22:22, 3 June 2016 (UTC)
Can someone from the project rescue the one-line stub Terbium difluoride? The editor specifically claims his incompetence on the topic and claims that the compound is "hypothetical" and "might exist" (see the initial stub he wrote). I placed a PROD tag but changed my mind after seeing that a few webpages at least seem to confirm the existence of the molecule. Help would be appreciated so thanks in advance! Pichpich (talk) 22:26, 11 June 2016 (UTC)
- That kind of stuff should be removed but it is laborious deleting articles in Wikipedia. So just ignore it and leave it bare. --Smokefoot (talk) 22:59, 11 June 2016 (UTC)
- Perhaps prod is a good way to deal with it. After looking on Google Scholar I find OCR errors, and one 10.1103/PhysRevLett.31.160 that should have read TbFe2 in its abstract. 10.1063/1.1702768 mentions TbF2 in its abstract, but no mention of anything in the body of the article, suggesting they never really made it. "MASS SPECTRA OF RARE EARTH β-DIKETONE COMPLEXES" mentions it as a mass spectroscopy fragment. https://www.physik.uni-hamburg.de/services/biblio/dissertation/dissfbPhysik/___Volltexte/Axel___Schnellbuegel/Axel___Schnellbuegel.pdf claims that the TbF2 does not exist due to energy of disproportionation. So I would say this is not notable, even if it could exist as an ion or molecule in isolation. Graeme Bartlett (talk) 00:17, 12 June 2016 (UTC)
- I've found one paper claiming to have made it (doi:10.1063/1.1702768) but I can't get into it and I suspect they're just using a short-hand in the abstract. You wouldn't think it a likely compound, Tb is one of the hardest Ln to get to a 2+ state, it's actually easier to get it to 4+ as that gets you an f half-shell. For example TbF4 has been known for 50 years (doi:10.1021/ja01642a001) . --Project Osprey (talk) 09:31, 13 June 2016 (UTC)
- I checked the full paper for the first DOI you mentioned (printed original, not subject to digitization mistakes). The abstract does indeed say TbF2 and that's not inconsistent with the rest of the article. But the article does not make further mention of this specific example (nor of many of the others listed in the abstract), and does not have any supporting data for any of the compounds. I don't see notability enough to merit an article for this compound. If anyone wants to track down refs cited by that lead ref, I can post them. Presumably various citation databases could uncover anything citing it. DMacks (talk) 21:13, 13 June 2016 (UTC)
- I was referring to (doi:10.1063/1.1702768, the "first" mntioned by Project Osprey), I agree I should have subscripted the 2 (as "TbF2"). @Graeme Bartlett: could you clarify which "first paper" you are discussing in this next comment about TbFe2? DMacks (talk) 16:32, 14 June 2016 (UTC)
- The first paper (10.1103/PhysRevLett.31.160 sorry we are talking about different papers) should really have said TbFe2, as you can see in the opening sentence, and confirmed in the reference it uses at 10.1103/PhysRevLett.29.1562. We could probably do with an article on TbFe2. It has a CAS number according to List of CAS numbers by chemical compound. Graeme Bartlett (talk) 09:19, 14 June 2016 (UTC) (In 10.1063/1.1702768 the F2 might be an error in place of F3.) Graeme Bartlett (talk) 01:59, 15 June 2016 (UTC)
- I added that, but be careful; CAS lists plenty of things that don't exist. A CASNo look-up through Scifinder give 16 refs: 8 of these are patents (mostly for hypothetical magnets), 6 are computational chem papers looking at Ln2+ in general, 1 is included in error (looking at ZnS:TbFx), that leaves one ref which I mentioned above. There's not much info there to make a page from, all we really have is "you can probably make it in the gas phase, but its probably not very stable". --Project Osprey (talk) 10:16, 14 June 2016 (UTC)
- I checked the full paper for the first DOI you mentioned (printed original, not subject to digitization mistakes). The abstract does indeed say TbF2 and that's not inconsistent with the rest of the article. But the article does not make further mention of this specific example (nor of many of the others listed in the abstract), and does not have any supporting data for any of the compounds. I don't see notability enough to merit an article for this compound. If anyone wants to track down refs cited by that lead ref, I can post them. Presumably various citation databases could uncover anything citing it. DMacks (talk) 21:13, 13 June 2016 (UTC)
- I've found one paper claiming to have made it (doi:10.1063/1.1702768) but I can't get into it and I suspect they're just using a short-hand in the abstract. You wouldn't think it a likely compound, Tb is one of the hardest Ln to get to a 2+ state, it's actually easier to get it to 4+ as that gets you an f half-shell. For example TbF4 has been known for 50 years (doi:10.1021/ja01642a001) . --Project Osprey (talk) 09:31, 13 June 2016 (UTC)
- Perhaps prod is a good way to deal with it. After looking on Google Scholar I find OCR errors, and one 10.1103/PhysRevLett.31.160 that should have read TbFe2 in its abstract. 10.1063/1.1702768 mentions TbF2 in its abstract, but no mention of anything in the body of the article, suggesting they never really made it. "MASS SPECTRA OF RARE EARTH β-DIKETONE COMPLEXES" mentions it as a mass spectroscopy fragment. https://www.physik.uni-hamburg.de/services/biblio/dissertation/dissfbPhysik/___Volltexte/Axel___Schnellbuegel/Axel___Schnellbuegel.pdf claims that the TbF2 does not exist due to energy of disproportionation. So I would say this is not notable, even if it could exist as an ion or molecule in isolation. Graeme Bartlett (talk) 00:17, 12 June 2016 (UTC)
I guess that there are more of this nearly-hypothetical compounds throughout the periodic table - where most do not warrant any article by themselves (as would be my judgement for this one). Making a rough split, 'binary halides of transition metals' and 'binary halides of lanthanides and actinides' might make two articles, where most of those could be listed (similarly one could consider for oxides/sulfides, and possibly carbides or nitrides), and incorporate (and redirect) all of these there. One could also park there the ones which are a bit more real, but not particularly notable (lower notability threshold: at least have references describing (computational) properties; higher notability threshold "real life 'use' (if only to chemists)"). --Dirk Beetstra T C 12:05, 14 June 2016 (UTC)
Merge Macrogol into Polyethylene glycol?
Comments at Talk:Polyethylene glycol#Merge Macrogol? would be welcome. --ἀνυπόδητος (talk) 12:48, 22 June 2016 (UTC)
I was looking at the double bond article and noticed a table with links for O=O and S=S. Since both are diradical ground states, I was wondering about this being misleading then went to the disulfur article and found the image at right in the infobox. Can anyone provide something more suitable and chemically accurate? Perhaps a space filling model, or something akin to the approach taken at oxygen where spectral lines and a discharge tube are shown? Thanks, EdChem (talk) 09:51, 9 July 2016 (UTC)
- Maybe I have forgotten some basics, but why dont we show O=O? The molecular graphics images of very simple molecules are not useful, perhaps replace them with Chemdraw, e.g. S=S. Oh, but even N2 structure has been shunted away from the Chembox. What's that all about? We might move it back and see what others say.--Smokefoot (talk) 12:39, 9 July 2016 (UTC)
5F-AMB structure
File:5F-AMB structure.png is used to illustrate the structure of this compound but has an (S) included indicating stereochemistry, but appearing like a sulfur atom. Would someone be willing to upload a new image (SVG, perhaps?) either without the (S) or with it not over the structure? Thanks, EdChem (talk) 14:26, 1 August 2016 (UTC)
- Same uploader also contributed File:5F-AMB-PICA.svg that has no stereochem label to obscure the structure. DMacks (talk) 15:04, 1 August 2016 (UTC)
- Oops, no, that's not the same compound (indole ≠ indazole). DMacks (talk) 15:11, 1 August 2016 (UTC)
- On a related note, I've added the CAS No for the S-isomer as that seems to be what the page is discussing. --Project Osprey (talk) 15:17, 1 August 2016 (UTC)
- Oops, no, that's not the same compound (indole ≠ indazole). DMacks (talk) 15:11, 1 August 2016 (UTC)
- @Aethyta: we're talking about your image. DMacks (talk) 15:17, 1 August 2016 (UTC)
- Thanks, I've switched my editor to the proper WP:MOSCHEM guidelines since then obviously, I've just uploaded a better image. But seriously, one of you could have done that already instead of discussing, it only takes a minute or two to draw and upload the structure. Aethyta (talk) 00:30, 2 August 2016 (UTC)
- I actually can't do SVG easily:( DMacks (talk) 02:10, 2 August 2016 (UTC)
- Sorry, didn't mean to be offensive. Do you have access to a computer/laptop running Windows, Linux or Mac OS? ChemAxon's MarvinSketch is free and does svgs just fine. You can copy paste import IUPACs, InChIs, smiles, even most CAS numbers, really amazing. If configured correctly (hehe) the resulting structure looks near identical compaired to chemdraw. Aethyta (talk) 04:12, 2 August 2016 (UTC)
- I actually can't do SVG easily:( DMacks (talk) 02:10, 2 August 2016 (UTC)
- Thanks, I've switched my editor to the proper WP:MOSCHEM guidelines since then obviously, I've just uploaded a better image. But seriously, one of you could have done that already instead of discussing, it only takes a minute or two to draw and upload the structure. Aethyta (talk) 00:30, 2 August 2016 (UTC)
Good Article Reassessment of Calcium chloride
Calcium chloride, an article that you or your project may be interested in, has been nominated for an individual good article reassessment. If you are interested in the discussion, please participate by adding your comments to the reassessment page. If concerns are not addressed during the review period, the good article status may be removed from the article. BlueMoonset (talk) 04:42, 19 August 2016 (UTC)
Template:Piperazines has been nominated for deletion. WikiProject Chemicals participants may be interested in contributing to the discussion at Wikipedia:Templates_for_discussion/Log/2016_August_29#Template:Piperazines. -- Ed (Edgar181) 19:01, 30 August 2016 (UTC)
Notice to participants at this page about adminship
Many participants here create a lot of content, have to evaluate whether or not a subject is notable, decide if content complies with BLP policy, and much more. Well, these are just some of the skills considered at Wikipedia:Requests for adminship.
So, please consider taking a look at and watchlisting this page:
You could be very helpful in evaluating potential candidates, and even finding out if you would be a suitable RfA candidate.
Many thanks and best wishes,
Anna Frodesiak (talk) 03:43, 1 September 2016 (UTC)
FAC nomination of β-hydroxy β-methylbutyric acid - need feedback on the article's "Chemistry" section
Would anyone here be willing to do a review of the "Chemistry" section of β-hydroxy β-methylbutyric acid at its FAC nomination? One reviewer there (Nergaal) suggested that a chemist or someone with a strong chemistry background needs to look at and review that section. I'd really appreciate it if one of you takes on a review at the FAC nomination or even just offers some constructive, actionable feedback of the article's chemistry section. Seppi333 (Insert 2¢) 22:07, 9 September 2016 (UTC)
MP/BP of β-Hydroxy β-methylbutyric acid
I've got a quick question about the melting point of β-Hydroxy β-methylbutyric acid (aka 3-Hydroxyisovaleric acid / β-Hydroxyisovaleric acid). I've found 3 sources† that list different MPs and BPs for the compound:
- the Human Metabolome Database (HMDB) lists the experimental MP as "65 - 67 °C"
- the Material Safety Data Sheet (which is hosted on the HMDB website) lists the MP as "−80 °C" and the BP as "88 °C / 1 mmHg"
- Chemspider lists the experimental BP (properties tab - under experimental data) as "128 °C / 7 mm (302.808 °C / 760 mmHg)"
Of these sources, only chemspider cites another source; hence, I assume the BP of 302.808 °C @ 760 mmHg is correct, but I'm still not really sure which MP to use. I was wondering if any of you knew of any database sources that corroborate the MP values (the only databases that I've checked are the ones currently listed in the article's drugbox); if not, should I just forego adding a MP to the drugbox?
- †Note that while the name listed in the compound heading/title differs with each source, they all list the same and CAS RN (625-08-1 ) and include the names from the other monographs as synonyms.
- @Edgar181: You responded to a similar concern which was raised at Talk:Beta-Hydroxy_beta-methylbutyric_acid/Archive_1#Melting_point a while back, so I figured I'd ping you in the event that you have any further thoughts about this.
Seppi333 (Insert 2¢) 19:11, 10 August 2016 (UTC)
- Not to address your request for information on the m.p., but I will say that quoting the b.p. is semi-useless or maybe worse. Such compounds (beta-hydroxy acids) cannot be distilled, they just wipe out. A lot of sources quote calculated b.p.'s, but I dont know if these numbers have any significance in describing the compound, and in fact almost propagate the false ideas. Maybe I am off base, in which case others might help illuminate me. --Smokefoot (talk) 07:26, 11 August 2016 (UTC)
- Smokefoot makes a good point. The 303 °C boiling point is not real - the compound will almost surely decompose well below temperature. Chemspider reports the experimental boiling point as "128 deg C / 7 mm (302.808 °C / 760 mmHg)". What this means is that it was experimentally determined to be 128 °C at reduced pressure, 7 mmHg. This can be extrapolated to estimate a boiling point at atmospheric pressure, 760 mmHg. But these kinds of estimates are not always very accurate and are have little real world meaning if the compound decomposes below that temperature anyway. It would be best to report just the experimental value (128 °C at 7 mmHg). With contradictory melting points, I'm not sure what is best to do. Maybe list multiple values with their references and let the reader decide what it means. Maybe just leave it out unless someone can find a reliable primary source experimental report rather than a database listing that might be erroneous and perpetuated from one database to another. For what it's worth, the source that I trust most, Chemical Abstracts, gives a mp value of "<-32 °C" with a reference that says, "PhysProp data were obtained from Syracuse Research Corporation of Syracuse, New York (US)". -- Ed (Edgar181) 20:28, 11 August 2016 (UTC)
- Ah, I didn't realize the 720 mmHg value was extrapolated from the BP @ 7 mmHg. Thanks for clarifying that; I'll tweak the BP in the infobox accordingly. I noticed that ChemSpider also states "MP (exp database): <-32 deg C" in the Predicted - EPIsuite tab under the Properties tab, so I suppose I'll add that to the drugbox.
I appreciate the assistance with resolving this. Seppi333 (Insert 2¢) 23:25, 11 August 2016 (UTC)
- Ah, I didn't realize the 720 mmHg value was extrapolated from the BP @ 7 mmHg. Thanks for clarifying that; I'll tweak the BP in the infobox accordingly. I noticed that ChemSpider also states "MP (exp database): <-32 deg C" in the Predicted - EPIsuite tab under the Properties tab, so I suppose I'll add that to the drugbox.
- Smokefoot makes a good point. The 303 °C boiling point is not real - the compound will almost surely decompose well below temperature. Chemspider reports the experimental boiling point as "128 deg C / 7 mm (302.808 °C / 760 mmHg)". What this means is that it was experimentally determined to be 128 °C at reduced pressure, 7 mmHg. This can be extrapolated to estimate a boiling point at atmospheric pressure, 760 mmHg. But these kinds of estimates are not always very accurate and are have little real world meaning if the compound decomposes below that temperature anyway. It would be best to report just the experimental value (128 °C at 7 mmHg). With contradictory melting points, I'm not sure what is best to do. Maybe list multiple values with their references and let the reader decide what it means. Maybe just leave it out unless someone can find a reliable primary source experimental report rather than a database listing that might be erroneous and perpetuated from one database to another. For what it's worth, the source that I trust most, Chemical Abstracts, gives a mp value of "<-32 °C" with a reference that says, "PhysProp data were obtained from Syracuse Research Corporation of Syracuse, New York (US)". -- Ed (Edgar181) 20:28, 11 August 2016 (UTC)
Addressed issue with images
| |||||
|---|---|---|---|---|---|
|
Images
Would anyone be willing to redraw File:Calcium hydroxymethylbutyrate skeletal.svg and File:Hydroxymethylbutyric acid.png in svg so that the lines in these diagrams and the placement of the hydroxy group are consistent with how it's drawn in File:HMB synthesis.svg? I nominated this article at FAC so I figured it'd be best if the structure diagrams for HMB were uniform throughout the article. Seppi333 (Insert 2¢) 00:23, 20 August 2016 (UTC)
|
Cocamidopropyl betaine
There are two areas were we could use help for Cocamidopropyl betaine, having to due with how we discuss and label it's production. First, the straightforward need to properly source and present how it is produced. We're currently linking to Organic synthesis, which appears to need large amounts of work itself. I'm wondering it might be better not to link to Organic synthesis, but a better quality article on the same topic, as Organic synthesis appears to be more than a bit of a POVFork. I assume there are standard, reliable sources available for how it is produced that editors that frequent this talk page would know.
Second, this chemical is apparently identified as an acceptable ingredient for "natural" products, and there is some dispute over whether or not it should be. This dispute may be nothing more than opposing marketing strategies. No sources have been offered to verify any of this, and the ip that repeatedly brought it up is now focusing on the production aspect instead.
So, we could use help with using proper terminology in the article, linking to proper related articles, and finding sources, especially for how cocamidopropyl betaine is produced. --Ronz (talk) 17:52, 9 September 2016 (UTC)
- I have added a better reference for the industrial process of making cocamidopropyl betaine from coconut oil. Whether this chemical is properly classified as "natural" or "synthetic" is not terribly important in my opionon - a lot of baggage is generally implied when people use those terms (especially in light of the common misconception that anything natural is inherently good and anything synthetic is inherently bad). Cocamidopropyl betaine is not found it nature, so in the strictest sense calling it natural would generally be misleading and calling it synthetic would be accurate. Whether it is natural or synthetic is irrelevant to its physical and chemical properties, though, so from a strictly chemical perspective it would be best not to use either term. But to the general reader who might be interested in whether an ingredient in their cosmetics is natural or synthetic, it could very well be an important characteristic. Regarding the article Organic synthesis, currently its focus seems to be more on the laboratory side of organic synthesis rather than the industrial side of organic synthesis (I assume that's what you mean by it appearing to be a POVFork) but it is an appropriate article to link to in my opinion. Chemical process or chemical synthesis might be other options to consider.-- Ed (Edgar181) 00:15, 10 September 2016 (UTC)
- Very much appreciated! --Ronz (talk) 15:13, 10 September 2016 (UTC)
{{Hydrides by group}}
For whatever reasons, an editor has started adding the template {{Hydrides by group}} to all hydrocarbons. That would be a lot of articles. Is this template helpful for readers? My recommendation is that articles be kept austere of tangential information for these reasons: 1) Readers seeking info about 1-octene is extremely unlikely to want to see the periodic table of hydride chemistry (CuH, TiHx etc). 2) Wikipedia is not a textbook. 3) What next? A template for carbon compounds? A separate template for alkenes? One for amines. So for an organometallic cmpd or normally complex natural product, the bottom of the article would have say 10 templates? So the community might think this through. --Smokefoot (talk) 13:13, 22 September 2016 (UTC)
- I don't think it makes sense to include a long list of alkanes, alkenes, and alkynes on a template for Binary compounds of hydrogen. Just one link to hydrocarbons would be enough. I agree that putting this template on individual articles for hydrocarbons is completely out of place - inorganic hydrides and hydrocarbons are so different chemically that there is no useful purpose served by including a template for navigating between such disparate topics. I recommend trimming the template and removing it from individual articles about hydrocarbons. -- Ed (Edgar181) 13:25, 22 September 2016 (UTC)
- Not planning on adding it to absolutely all hydrocarbons - I can see the obvious problems there. That's why I've only included up to 20 for only three categories of hydrocarbons.
- Even at that, it probably shouldn't be removed form absolutely everything. Methane, ethane, propane and butane should probably keep it, but I could definitely see it being removed from pentane and upwards. - AwesoMan3000 (talk) 15:55, 22 September 2016 (UTC)
- Offhand, I can't think of many situations in which methane acts as a hydride. Deep space perhaps? Although that might just be some sort of crazy photolysis product. In any even it seems very fringe. --Project Osprey (talk) 17:02, 22 September 2016 (UTC)
- Well, the template idea was well intended but the result potentially very confusing.--Smokefoot (talk) 23:02, 22 September 2016 (UTC)
- It should stick to the emphasis on groups, and not get carried away with hydrocarbons. Perhaps hydrocarbons could be split to another template just for them. Graeme Bartlett (talk) 05:10, 23 September 2016 (UTC)
- I've only listed the first 10 straight alkanes and first 9 of each alkenes and alkynes, and taken it off of any longer ones. - AwesoMan3000 (talk) 08:27, 23 September 2016 (UTC)
- It should stick to the emphasis on groups, and not get carried away with hydrocarbons. Perhaps hydrocarbons could be split to another template just for them. Graeme Bartlett (talk) 05:10, 23 September 2016 (UTC)
- Well, the template idea was well intended but the result potentially very confusing.--Smokefoot (talk) 23:02, 22 September 2016 (UTC)
- Offhand, I can't think of many situations in which methane acts as a hydride. Deep space perhaps? Although that might just be some sort of crazy photolysis product. In any even it seems very fringe. --Project Osprey (talk) 17:02, 22 September 2016 (UTC)
I've added labels to the images at Template:Hydrides by group. Currently H2 and HD are listed as hydrogen halides, which they aren't. Should either of these be included at all, given binary hydrides implies hydrogen with some other element? Also, any reason for separating borane and diborane under "boranes" but away from all the other boranes? Thoughts? EdChem (talk) 06:10, 23 September 2016 (UTC)
- They don't really seem to follow a general formula like the alkanes, azanes, etc. I could categorise them all under the Boranes section but that might look a bit messy IMO. - AwesoMan3000 (talk) 08:27, 23 September 2016 (UTC)
- Placed as they are at present, I think many readers would wonder if they aren't boranes. They are boranes. I think there is potential to confuse / mislead. EdChem (talk) 12:19, 23 September 2016 (UTC)
It's worth noting that hydrocarbons are unlike any other hydrides on this list, in that Carbon is more electronegative than Hydrogen. As such you basically never see hydride abstraction, but you do get addition or substitution reactions instead. Take the simple reaction of a hydride with an acid: methane will react with superacids to give Methanium, which is essentially stable (under the extreme condition at which it is formed). You don't see the abstraction reaction:
CH4 + H+ → CH3+ + H2
As such I don't think they belong on this list, as they don't display hydride chemistry. I understand why they were added, it fills in a black area of a table, which is something that all scientists find very appealing. Unfortunately nature doesn't always conform to well ordered lists and these materials do not belong - or if we are going to include them we should probably explain ourselves somewhere. --Project Osprey (talk) 09:40, 23 September 2016 (UTC)
- The text at Hydride#Types of hydrides includes discussion of whether alkanes are hydrides in a technical sense. Note that this "hydrides" template is headed and links to binary compounds of hydrogen, which definitely does include hydrocarbons. Maybe we need to decide which this is. EdChem (talk) 12:25, 23 September 2016 (UTC)
- The template was originally created just to link the pages which are currently on the left of the template. And typing "hydrides" is a lot easier than typing "binary compounds of hydrogen".
- Maybe we should move it to something like "Binary hydrides"? - AwesoMan3000 (talk) 12:31, 23 September 2016 (UTC)
- It seems that there is some research/homework project going on. Otherwise, I dont get it. Categories are being applied that are not needed, are controversial, potentially misleading to nonchemists, etc. There are more useful things to do around here than trying to shoe-horn various compounds into one's notions of categories. --Smokefoot (talk) 13:17, 23 September 2016 (UTC)
3-Methylcyclopropene
This article is nominated for deletion at Wikipedia:Articles for deletion/3-Methylcyclopropene. Contribution from participants of this WikiProject would be helpful. ChemNerd (talk) 14:39, 23 September 2016 (UTC)
- It was converted into a redirect. --Leyo 15:41, 10 October 2016
Space-filling models of S2 and C2
I'm not sure if this should have been posted some place on Wikimedia Commons, but I don't think there's a place to request files of models of molecules, so I'll out this here instead.
Reviving an archived discussion: the file for disulfur is horribly inconsistent with all the other homonuclear diatomic molecules, which have space-filling models compared to this weird ball-and-stick thing.
On a similar note, no 3D model seems to exist for diatomic carbon. Could one be made? I don't have the neccesary programs.
- AwesoMan3000 (talk) 17:16, 21 September 2016 (UTC)
- There is little point for any electronic drawing for diastomic species. What insight/info would such a picture provide?--Smokefoot (talk) 13:13, 22 September 2016 (UTC)
- I think the better question is whether this image for disulfur is misleading and whether it could be replaced with anything better. Maybe something like File:Dioxygen-3D-vdW.png but in yellow? EdChem (talk) 06:13, 23 September 2016 (UTC)
- Does disulfur even have a double bond? I'm pretty sure that diatomic oxygen is actually a radical with only a single bond, and with sulfur being a heavier analogue of oxygen I'd predict they'd show some form of similarity. - AwesoMan3000 (talk) 11:10, 28 September 2016 (UTC)
- I believe so. The ground state for S2 is 3Σg-, the same as for O2 - so you would expect a double bond with a di-radical in the triplet state. However sulfur is group 3 so spin-orbit effects are much larger, making it more reactive in various ways. Thing is, as Smokefoot pointed out, it's pretty much impossible to explain any of this with ball and stick model. You'd need a molecular orbital diagram. --Project Osprey (talk) 13:45, 28 September 2016 (UTC)
- The space-filling model doesn't really show bonds (at elast not directly), which probably makes matters even less confusing. - AwesoMan3000 (talk) 06:39, 3 October 2016 (UTC)
- I believe so. The ground state for S2 is 3Σg-, the same as for O2 - so you would expect a double bond with a di-radical in the triplet state. However sulfur is group 3 so spin-orbit effects are much larger, making it more reactive in various ways. Thing is, as Smokefoot pointed out, it's pretty much impossible to explain any of this with ball and stick model. You'd need a molecular orbital diagram. --Project Osprey (talk) 13:45, 28 September 2016 (UTC)
- Does disulfur even have a double bond? I'm pretty sure that diatomic oxygen is actually a radical with only a single bond, and with sulfur being a heavier analogue of oxygen I'd predict they'd show some form of similarity. - AwesoMan3000 (talk) 11:10, 28 September 2016 (UTC)
- I think the better question is whether this image for disulfur is misleading and whether it could be replaced with anything better. Maybe something like File:Dioxygen-3D-vdW.png but in yellow? EdChem (talk) 06:13, 23 September 2016 (UTC)
Do we really want to have such articles with three chemboxes of different isomers? --Leyo 08:45, 4 October 2016 (UTC)
- A bit too crazy, no content, just chemboxes. I've moved the boxes to the talkpage for reference, and turned it more into a 'disambig'-like article to show the three different isomers. --Dirk Beetstra T C 08:51, 4 October 2016 (UTC)
- Ah, it is the skeleton that one encounters in some natural compounds, maybe that gives the article enough notability (a couple of references like doi:10.1016/0031-9422(96)00121-5). --Dirk Beetstra T C 08:53, 4 October 2016 (UTC)
- Whatever it may be, antiimplantation drug with a hydrogenated benzoxepin skeleton - 10.2174/1570180053398253. --Dirk Beetstra T C 08:56, 4 October 2016 (UTC)
- BTW: de.wikipedia has an article on 3-benzoxepin. --Leyo 09:23, 4 October 2016 (UTC)
- @Leyo: I have translated it into 3-benzoxepin. Needs language cleanup and some link updates. --Dirk Beetstra T C 11:14, 4 October 2016 (UTC)
- Thanks, but could you please check the article title? --Leyo 13:24, 4 October 2016 (UTC)
- Hmm .. missing dash, and something else .. am I overlooking something? --Dirk Beetstra T C 13:29, 4 October 2016 (UTC)
- That was all. ;-) May the redirect created by the move get deleted? BTW: fr:Benzoxépine contains some content about the tautomerism. --Leyo 14:52, 4 October 2016 (UTC)
- Leave the redirect, not too crazy to use a space. My French is not that good, but I'll have a look. --Dirk Beetstra T C 15:48, 4 October 2016 (UTC)
- That was all. ;-) May the redirect created by the move get deleted? BTW: fr:Benzoxépine contains some content about the tautomerism. --Leyo 14:52, 4 October 2016 (UTC)
- Hmm .. missing dash, and something else .. am I overlooking something? --Dirk Beetstra T C 13:29, 4 October 2016 (UTC)
- Thanks, but could you please check the article title? --Leyo 13:24, 4 October 2016 (UTC)
- @Leyo: I have translated it into 3-benzoxepin. Needs language cleanup and some link updates. --Dirk Beetstra T C 11:14, 4 October 2016 (UTC)
- BTW: de.wikipedia has an article on 3-benzoxepin. --Leyo 09:23, 4 October 2016 (UTC)
Big data improvement in Wikidata
Just to announce you than thanks to the work of @Sebotic:, Wikidata increases its coverage of chemicals with a total of ~98'000 chemicals having an item. All data were imported from PubChem and ChEBI and respect the rules about sources leading to a high improvement of the data quality. Right now an important step has to start to curate the data especially to merge duplicated items. You are welcome to take part to this action and you can get in touch with the Chemistry project in WD for details.
From that work additional importations can start in order to add more identifiers but please announce your intention of data import before any huge importation in order to coordinate the work of bots and of contributors curating the conflicts. Snipre (talk) 10:09, 12 October 2016 (UTC)
Bunch of ELs being added to industrialchemistry.org
- industrialchemistry.org: Linksearch en (insource) - meta - de - fr - simple - wikt:en - wikt:fr • Spamcheck • MER-C X-wiki • gs • Reports: Links on en - COIBot - COIBot-Local • Discussions: tracked - advanced - RSN • COIBot-Link, Local, & XWiki Reports - Wikipedia: en - fr - de • Google: search • meta • Domain: domaintools • AboutUs.com
See Special:Contributions/Raffinate2 -bunch of recent edits like this. Pinging User:Raffinate2 and asking folks here what they think. Jytdog (talk) 08:48, 8 October 2016 (UTC)
- @Jytdog: Hmm .. and what is that adding over what is already in those articles? --Dirk Beetstra T C 10:36, 12 October 2016 (UTC)
- Yep that's what led me to post here. Maybe folks here find it valuable; seems kind of spammy to me. Jytdog (talk) 17:05, 12 October 2016 (UTC)
- The data is a good resource, but the pages contain all that info already. I do think that the additions were certainly spammy, even though the material itself is not. I have removed them all. --Dirk Beetstra T C 03:45, 13 October 2016 (UTC)
- Yep that's what led me to post here. Maybe folks here find it valuable; seems kind of spammy to me. Jytdog (talk) 17:05, 12 October 2016 (UTC)
Something seems to be wrong since the infobox of both compounds contains the CAS RN 91449-79-5. If a correction is made, it needs to be done in the corresponding Wikidata item, too. --Leyo 12:07, 1 October 2016 (UTC)
- Should I rather add {{Merge}}? --Leyo 07:30, 24 October 2016 (UTC)
Missing CAS numbers
I was unable to find the CAS numbers for the following compounds:
Does anyone have access to SciFinder? --Leyo 08:39, 2 November 2016 (UTC)
- Done for Lugdunin and PZM21 but I can't track one down for YInMn Blue. Regarding PZM21, I think its WP:TOOSOON and should probably be deleted. There's one paper on it with other groups apparently taking issue with its conclusions (see here doi:10.1038/nature19424 and here doi:10.1021/acschemneuro.6b00167) --Project Osprey (talk) 13:37, 2 November 2016 (UTC)
- Thank you. --Leyo 19:30, 2 November 2016 (UTC)
Does polybutyrate have a CAS RN at all? --Leyo 15:36, 3 November 2016 (UTC)
- 60961-73-1 --Project Osprey (talk) 15:44, 3 November 2016 (UTC)
Major confusion between stereoisomers
Hi, I am having a problem with this image and the images I made that were based on it. I am not sure if it is accurate, but I am equally unsure of the correct names for these stereoisomers.
See the relevant talk page for more details.
- Jynto (talk) 16:34, 3 November 2016 (UTC)
- Hi there. It looks like I drew that image, but it was nearly ten years ago now so not sure where I got it from! I would guess it was derived from one of my med chem textbooks, when I have time I will have a look through them and see if I can verify which ones are which. It may well be incorrect though if it doesn't match the pages for the individual compounds, I made a few mistakes of that kind back in those days. Meodipt (talk) 02:52, 4 November 2016 (UTC)
PD-chem template
As part of expanding the recent aluminium triacetate article, I have uploaded File:CaAl(OH)(H2O)Az2 dihydrate.png as an image of one hypothesised structure formed when aluminium triacetate acts as a mordant with alizarin. The image looks poor, so if someone would re-draw it as an .SVG file, that would be excellent. Also, I have found that I could not tag it with the usual PD-chem template as it has been deleted. I have requested it be restored at Wikipedia:Requests_for_undeletion#Template:PD-chem and invite comment from anyone with a view on it being restored or remaining deleted. I have also posted to WT:CHEMISTRY. EdChem (talk) 01:43, 23 November 2016 (UTC)
- It's back. But commons could well be the pace for your image. Graeme Bartlett (talk) 02:40, 23 November 2016 (UTC)
A request for comment has been made at the above link. Your input is welcome. Boghog (talk) 16:46, 27 November 2016 (UTC)
A request for comment has been made at the above link. Your input is welcome. Boghog (talk) 11:51, 30 October 2016 (UTC)
- Do like you want in en:WP but please don't create a new item for this new article. Thanks Snipre (talk) 21:21, 31 October 2016 (UTC)
- I am assuming you are referring to Wiki Data. Testosterone was temporarily made into a disambiguation page that linked to Testosterone (hormone) and Testosterone (medication). The page view statistic were 5:1 in favor of the hormone, hence it was clear that the hormone is the primary topic. Subsequently Testosterone (hormone) was moved back to Testosterone and the disambiguation page was moved to Testosterone (disambiguation). We also have the following Wikidata items:
- testosterone (Q1318776) (chemical compound/hormone)
- testosterone (Q27863114) (therapeutic use)
- I have no opinion on whether having two Wikidata items for testosterone this is a good or a bad thing. Boghog (talk) 17:05, 27 November 2016 (UTC)
- I am assuming you are referring to Wiki Data. Testosterone was temporarily made into a disambiguation page that linked to Testosterone (hormone) and Testosterone (medication). The page view statistic were 5:1 in favor of the hormone, hence it was clear that the hormone is the primary topic. Subsequently Testosterone (hormone) was moved back to Testosterone and the disambiguation page was moved to Testosterone (disambiguation). We also have the following Wikidata items:
SMILES of trans-Bicalicene
c1c/c/2c\3/c(c3)c4/c(c/5\c(c2c1)c5)/ccc4 is a SMILES of trans-Bicalicene. But, Jmol can't recognize c1c/c/2c\3/c(c3)c4/c(c/5\c(c2c1)c5)/ccc4 as an aromatic molecule.[1] --Abelium (talk) 16:01, 6 December 2016 (UTC)
2016 Community Wishlist Survey Proposal to Revive Popular Pages

Greetings WikiProject Chemicals/Archive 2016 Members!
This is a one-time-only message to inform you about a technical proposal to revive your Popular Pages list in the 2016 Community Wishlist Survey that I think you may be interested in reviewing and perhaps even voting for:
If the above proposal gets in the Top 10 based on the votes, there is a high likelihood of this bot being restored so your project will again see monthly updates of popular pages.
Further, there are over 260 proposals in all to review and vote for, across many aspects of wikis.
Thank you for your consideration. Please note that voting for proposals continues through December 12, 2016.
Best regards, Stevietheman — Delivered: 17:56, 7 December 2016 (UTC)
Need help
Hi, can someone give me some explanation about the differences between these two compounds: (Cyano-κC)potassium and [http://www.chemspider.com/Chemical-Structure.8681.html POTASSIUM CYANIDE ? Is it just a problem of structure drawing or the first compound is a real and stable compound ? Thanks Snipre (talk) 17:47, 6 December 2016 (UTC)
- I'm pretty sure this is a representational problem in ChemSpider; potassium won't form covalent bonds that easily, unless you're talking very unusual conditions of temperature, etc. ChemSpider often has two (or more!) structures for things that should be ionic, along these lines - it just depends how it was drawn in a website that ChemSpider pulled information from. I've asked User_talk:The_chemistds (who works at ChemSpider, I think) to comment. Walkerma (talk) 20:52, 6 December 2016 (UTC)
- Like Walkerma indicates, but stated more strongly: These drawings (K-CN) are the fiction that children are taught and some drawing programs produce. Utter nonsense. KCN is a 3-d polymer as a solid and in solution is an aquo complex of K+ and dissociated cyanide. I guess these pictures might approximate gas phase structures.--Smokefoot (talk) 23:16, 6 December 2016 (UTC)
- @Smokefoot and Walkerma: Thanks for your feedback but the problem is larger than ChemSpider: ChemIDplus has the same covalent structure and worst, they generated InChIKey from that wrong structure. Do you know a way to contact ChemIDplus team in order to report the error ? Snipre (talk) 12:08, 8 December 2016 (UTC)
- The issue is pervasive, and I dont think that purveyors of these programs really care. Professional chemists know that many drawing programs produce misrepresentations of many species. The further problem is that for inorganic salts, the structures change drastically in solution. --Smokefoot (talk) 14:22, 8 December 2016 (UTC)
- @Smokefoot and Walkerma: Thanks for your feedback but the problem is larger than ChemSpider: ChemIDplus has the same covalent structure and worst, they generated InChIKey from that wrong structure. Do you know a way to contact ChemIDplus team in order to report the error ? Snipre (talk) 12:08, 8 December 2016 (UTC)
- Like Walkerma indicates, but stated more strongly: These drawings (K-CN) are the fiction that children are taught and some drawing programs produce. Utter nonsense. KCN is a 3-d polymer as a solid and in solution is an aquo complex of K+ and dissociated cyanide. I guess these pictures might approximate gas phase structures.--Smokefoot (talk) 23:16, 6 December 2016 (UTC)
2016 Community Wishlist Survey - Proposals related to "Recent Changes"
Hello again WP Chemicals members! I also noticed that you make extensive use of RecentChangesLinked to produce change patrols in a "Recent changes" template. Here's a couple Wishlist Survey proposals related to making these patrols more productive:
- "Hide trusted users" checkbox option on watchlists and related/recent changes (RC) pages
- RecentChangesLinked should search associated pages
Please consider and vote on these by December 12. Thank you again for your time! Stevie is the man! Talk • Work 16:00, 8 December 2016 (UTC)
Molecule or salt ?
Which is the most correct structure for SeSn ? A covalent bond or a ionic bond ? See the table
| Database | Covalent | Ionic |
|---|---|---|
| Wikidata | Q6589976 | Q27283356 |
| PubChem | 6432049 | 9877645 |
| ChemSpider | [www.chemspider.com/Chemical-Structure.4937309.html 4937309] | [www.chemspider.com/Chemical-Structure.8053322.html 8053322] |
| InChI | 1S/Se.Sn | 1S/Se.Sn/q-2;+2 |
| InChIKey | MFIWAIVSOUGHLI-UHFFFAOYSA-N | LYZMBUYUNBCSMW-UHFFFAOYSA-N |
Snipre (talk) 15:45, 8 December 2016 (UTC)
- The pictures drawn by these sites are nonsense. Get over it or get used to it. There is a reason that inorganic chemistry is distinguished from organic - and your questions illustrate one aspect of the difference - the structural chemistry in inorganic is a whole new game compared to the world of most organic compounds, which mainly conforms to well defined (computer-definable) structural rules and is mainly comprised of molecular species with low lattice energies that retain their structures in the solid and in solution. Not to say that one area is more advanced or better than the other (or even completely distinct as illustrated by organometallic chemistry). --Smokefoot (talk) 17:52, 8 December 2016 (UTC)
- The structure would be a three dimensional lattice. Normally you would illustrate this with a diagram of the unit cell, and give the lattice parameters so you can tell how it fills space. Graeme Bartlett (talk) 21:41, 8 December 2016 (UTC)