Wikipedia:Reference desk/Archives/Science/2013 February 10
| Science desk | ||
|---|---|---|
| < February 9 | << Jan | February | Mar >> | February 11 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 10
[edit]Annihilation
[edit]Would an electron and positron annihilating be considered two particles occupying the same space?GeeBIGS (talk) 02:21, 10 February 2013 (UTC)
- Not after they were annihilated (which means they would have ceased to exist). -- Jack of Oz [Talk] 03:44, 10 February 2013 (UTC)
Well how about before or during then? Sounds like the same exact place to me....NOWHERE.GeeBIGS (talk) 06:20, 11 February 2013 (UTC)
- No, both electrons and positrons are fermions which, by the Pauli exclusion principle cannot occupy the same quantum state (the concept of "same space" at this scale is somewhat meaningless; we don't speak in terms of location, since these particles are not localizable, but instead we speak of particles occupying a certain "quantum state", which is functionally equivalent to "location" for the purpose of discussions like this). However, bosons can occupy the same quantum state as each other; this leads to materials like Bose–Einstein condensates. --Jayron32 04:40, 10 February 2013 (UTC)
- To my understanding, the Pauli exclusion principle only applies to the full quantum state of fermions. Any difference in quantum numbers will allow cohabitation - which is why you can get two electrons in one atomic orbital. They share quantum states except for one is "spin up" and the other is "spin down". I'm not familiar enough with QED to know the answer, but wouldn't the difference in charge on electrons and positrons be sufficient to introduce them into different quantum states, even if everything else about them was identical? If so, then the Pauli exclusion principle would say nothing about them regarding being able to occupy the same space or not. -- 205.175.124.30 (talk) 01:53, 11 February 2013 (UTC)
- Well, that's why I hedged over the use of the term "same space". What happens is their probability space overlaps as they get closer, and eventually the likelihood of annihilation becomes great enough to cause it to happen, and it does. They can do this without the "points" occupying the "same space", or even having the same quantum state. --Jayron32 04:09, 11 February 2013 (UTC)
- To my understanding, the Pauli exclusion principle only applies to the full quantum state of fermions. Any difference in quantum numbers will allow cohabitation - which is why you can get two electrons in one atomic orbital. They share quantum states except for one is "spin up" and the other is "spin down". I'm not familiar enough with QED to know the answer, but wouldn't the difference in charge on electrons and positrons be sufficient to introduce them into different quantum states, even if everything else about them was identical? If so, then the Pauli exclusion principle would say nothing about them regarding being able to occupy the same space or not. -- 205.175.124.30 (talk) 01:53, 11 February 2013 (UTC)
So how close do they get to each other before becoming empty space?68.36.148.100 (talk) 00:21, 11 February 2013 (UTC)
- Electrons (and positrons) have no physical size, so are always empty space. (However they do have electrical fields which extend for a distance.) There is no specific cutoff distance for when they annihilate - the probability just gets larger and larger the closer they are. They can even orbit each over for a while, see: Positronium. Ariel. (talk) 00:59, 11 February 2013 (UTC)
- Joke: how many electrons and positrons can you fit in a lightbulb? Infinity. But seriously, Could you give a little more in your explanation? Thanks.68.36.148.100 (talk) 01:32, 11 February 2013 (UTC)
- What do you want to know? Ariel. (talk) 03:01, 11 February 2013 (UTC)
- Joke: how many electrons and positrons can you fit in a lightbulb? Infinity. But seriously, Could you give a little more in your explanation? Thanks.68.36.148.100 (talk) 01:32, 11 February 2013 (UTC)
"their probability space overlaps as they get closer, and eventually the likelihood of annihilation becomes great enough to cause it to happen, and it does." I don't mean to sound rude , but this sound like some south Philly used car salesman triple talk to me. Ariel, could you please explain this to me in other words?GeeBIGS (talk) 06:15, 11 February 2013 (UTC)
- An electron does not have a defined position, but rather there is a chance of finding it anywhere. Technically it could even be light years away from where you would normally think of as it's "location" - but at an extremely low probability. That's what he means by "probability space". So when the electron and positron approach each other there is a chance that they actually happen to both be in the same space at the same time, but also a chance that they are not "near" each other. The closer they get the greater the chance of finding them both in the same spot. Once that happens they will annihilate. The visible effect of this is time. Instead of annihilating instantly they take some time to do it, and you can define a "half life" of sorts of how long it takes them to both randomly happen to be in the same place at the same time. But remember that it's random - for the same distance some will annihilate extremely fast, some will take longer. But you can calculate how long it will take half of them to annihilate. And of course the closer they are the shorter that time is - but it's never 0 and it's never infinity. I hope this helps. Ariel. (talk) 09:10, 11 February 2013 (UTC)
Define "in the same spot" and "the same place at the same time" and if you could please explain how both simultaneously ceasing to exist is not the same as being in the same space . GeeBIGS (talk) 12:00, 11 February 2013 (UTC)
- "the same place at the same time" means their waveforms overlap such that the waves exactly cancel out. You can (if you want) define "the same space" as the location of maximum probability. But it's not necessary for those two locations (as defined that way) to be the same for them to annihilate. Ariel. (talk) 19:01, 11 February 2013 (UTC)
- No, they don't cancel, since that would violate conservation of energy. There's no special rule about annihilation in quantum field theory. Decays of the form particle + its antiparticle → other particle + its antiparticle are allowed (if energy-momentum is conserved) simply because antiparticles have opposite conserved charges, so all the charges add up to 0 on both sides and hence are conserved in the decay. In particular, the photon is its own antiparticle, so particle + antiparticle → 2 photons is always possible (though it may not happen very often if the particle interacts with light only indirectly). -- BenRG (talk) 19:41, 11 February 2013 (UTC)
Mass of a test tube full of bare nuclei
[edit]I read this in a book A test tube full of bare nuclei will weigh heavier than the earth. If the test tube is heavier than earth, it means test tube contains more nuclei than the earth. So, tube is heavier than the earth. This also means that the density of test tube is more than the density of neutron star. This doesn't sound good to me, I think the fact (italic sentence) is wrong. What do you think about this? Show your knowledge (talk) 03:48, 10 February 2013 (UTC)
- Are you sure you have that quote right ? It's bad English. Proper English would be "A test tube full of bare nuclei will weigh more than the Earth" or "A test tube full of bare nuclei will be heavier than the Earth". StuRat (talk) 03:54, 10 February 2013 (UTC)
- I have written the same quote written in the book. Scientifically, I also think this quote is not right. What is your opinion about this? Show your knowledge (talk) 04:12, 10 February 2013 (UTC)
- A test tube full of bare nuclei would be functionally as dense as a Neutron star, which has a density (at the lower estimate) of 3.7×1017 kg/m3 which means that a test tube full (say 5 cubic centimeters) would have a mass of 3.7×1017 kg/m3 x 5×10-6 m3 = 1.85 ×1012 kg. The earth has a mass of 5.9736×1024 kg, so no, it would not weigh more than the earth. It's 12 orders of magnitude lighter than the earth. But it's still really freaking heavy; a Boeing 787 has a mass of about 2.27×105 kg; so our test tube full of nuclei would still be heavier than 9,000,000 jumbo jets. --Jayron32 04:34, 10 February 2013 (UTC)
- A typical test tube is actually more like 10cc's. But our Orders_of_magnitude_(mass) article (which is great for this kind of question) says that a teaspoonful (5ml) of neutron star material would have a mass of 5.5x1012...so I guess we might say 1013kg...but still nowhere near the mass of the earth. The orders of magnitude article suggest that a 1km tall mountain would have about the same mass as our test tube. About a thousand olympic-sized swimming pools full of nucleii would balance the mass of the Earth pretty well. SteveBaker (talk) 04:49, 10 February 2013 (UTC)
- 5.5... 1.85.... close enough for government work... ;) --Jayron32 05:06, 10 February 2013 (UTC)
- So that quote is both wrong and bad English. I think I'd avoid that author. StuRat (talk) 04:50, 10 February 2013 (UTC)
- I wonder if a pyrex test tube would be strong enough to contain a mass equivalent to millions of jet airplanes. ←Baseball Bugs What's up, Doc? carrots→ 05:32, 10 February 2013 (UTC)
- I wondered the same thing. I'm pretty sure the answer is no. That means we're talking about a strictly theoretical (= non-existent) test tube; and I don't see how we can have real nuclei sitting inside a non-existent test tube. So that must mean we're talking about theoretical (= non-existent) nuclei. In theory there's no difference between theory and practice; but in practice there kinda is, and this is a really good demonstration of it. -- Jack of Oz [Talk] 05:45, 10 February 2013 (UTC)
- Well, it's not just the mass. What's keeping them squoze together? If they're "bare nuclei" other than neutrons, then the whole thing is positively charged and is furiously repelling itself while attracting surrounding electrons; I don't think you'd survive being anywhere near. If they're neutrons, then they have a half-life of about 15 minutes, meaning roughly 10000 times as radioactive as the Polonium-210 a microgram or so of which killed Litvinenko (in terms of disintegrations per unit time; admittedly each disintegration might be lower energy, but when you've got 10^26 times more of them I doubt that's going to help much). --Trovatore (talk) 08:21, 10 February 2013 (UTC)
- Maybe only if it's really high quality pyrex. Although this strikes me as a half-cousin to the old question, "If you could develop a universal solvent, what would you store it in?" ←Baseball Bugs What's up, Doc? carrots→ 06:21, 10 February 2013 (UTC)
- I wondered the same thing. I'm pretty sure the answer is no. That means we're talking about a strictly theoretical (= non-existent) test tube; and I don't see how we can have real nuclei sitting inside a non-existent test tube. So that must mean we're talking about theoretical (= non-existent) nuclei. In theory there's no difference between theory and practice; but in practice there kinda is, and this is a really good demonstration of it. -- Jack of Oz [Talk] 05:45, 10 February 2013 (UTC)
- I wonder if a pyrex test tube would be strong enough to contain a mass equivalent to millions of jet airplanes. ←Baseball Bugs What's up, Doc? carrots→ 05:32, 10 February 2013 (UTC)
This is great, I got the answer to my question in 1 hour. But I have already posted a question two days before (8 Feb, heading: Symbol of mass number) still I don't have the clear answer of that question. Please, do something about that. Show your knowledge (talk) 05:13, 10 February 2013 (UTC)
- What's stopping you from googling it? ←Baseball Bugs What's up, Doc? carrots→ 05:32, 10 February 2013 (UTC)
- Sure, if you can get Wikipedia to double my pay for answering your questions, maybe I'll get on that. --Jayron32 05:34, 10 February 2013 (UTC)
- Jayron32's pay rate is hereby doubled, so he will henceforth be paid twice as much per answer to Wikipedia Q as he was previously. StuRat (talk) 05:47, 10 February 2013 (UTC)
- Strangely enough, my wallet doesn't seem any heavier. --Jayron32 06:01, 10 February 2013 (UTC)
- In that case, I'd better triple your pay rate. StuRat (talk) 06:04, 10 February 2013 (UTC)
- You'll get what's coming to you, I can guarantee you that, Jayron. -- Jack of Oz [Talk] 10:48, 10 February 2013 (UTC)
- That's always what I am afraid of... --Jayron32 18:26, 10 February 2013 (UTC)
- Isn't the issue here what exact meaning the word "full" has in this context? It talks about multiple nuclei, so that implies a least some spacing between them (if they were touching then wouldn't it just be one big nucleus?), and that spacing will by definiton determine the density. We normally say a test tube is full of water if any additional water would over flow, but we don't require the water to be compressed to qualify as being truly full. Conversely, a hydrogen cylinder is empty if it's pressure is equal to 1atm. 202.155.85.18 (talk) 00:31, 11 February 2013 (UTC)
- I believe we're talking about the maximum density of neutrons it's possible to achieve outside of a black hole. Similarly to the core of a neutron star where the neutrons can't get any closer together due to quantum degeneracy pressure (ie the Pauli exclusion principle). True, our OP might be thinking of a less dense packing of neutrons - but what we're saying is that even at the densest packing imaginable, the test tube still only weighs as much as Snowdon - a modest-sized mountain. SteveBaker (talk) 16:04, 11 February 2013 (UTC)
- Isn't the issue here what exact meaning the word "full" has in this context? It talks about multiple nuclei, so that implies a least some spacing between them (if they were touching then wouldn't it just be one big nucleus?), and that spacing will by definiton determine the density. We normally say a test tube is full of water if any additional water would over flow, but we don't require the water to be compressed to qualify as being truly full. Conversely, a hydrogen cylinder is empty if it's pressure is equal to 1atm. 202.155.85.18 (talk) 00:31, 11 February 2013 (UTC)
- That's always what I am afraid of... --Jayron32 18:26, 10 February 2013 (UTC)
- You'll get what's coming to you, I can guarantee you that, Jayron. -- Jack of Oz [Talk] 10:48, 10 February 2013 (UTC)
- I just realized...if you turned something with the mass of the Earth into a black hole, it would have a Schwartzchild Radius of about 1cm. That's a 2cm diameter - so at that density, it would just about fit into a large test tube...but not into a smaller one. SteveBaker (talk) 16:09, 11 February 2013 (UTC)
solar ac dc inverters failure
[edit]Is there a way to test an inverter to see if it is functioning properly, or do they just quit completely? My batteries are not lasting like they should and was told it could be the inverter. off grid207.212.113.253 (talk) 04:03, 10 February 2013 (UTC)
- As a general, but not absolute, rule, electronics tend to fail catastrophically, or fail with some part getting far too hot, emitting a "brown smell". So, if your invertor has not failed completely, is not showing any warning/fault display, and is not emitting burnt smells, it's most likely not your problem. You can test an invertor by testing first with no load, then with something (such as an electric room heater) that loads it to at or near its full rating. Measure the DC input current for both conditions, and comnpare with the manufactuer's data. Typically the DC input amps should be roughly 1 twentieth the rated load in watts divided by the battery voltage. The full load DC input amps should be about 1.1 times the actual load divided by the battery voltage. Do not be concerned if the no load DC amps varies somewhat from this.
- Are you aware that batteries are subject to both catastrophic failure and gradual loss in capacity? Battery life is dependent on brand quality, ambient temperature, the number of cycles, and the depth of discharge. In the hot climate regions of Australia, battery life for cheap European lead acid batteries can be as short as two summers. A life of ten years for a generously sized quality Janpanese battery is a good achievement. The output of the cheaper solar panels also deteriorates over time. A panel service life to 50% output of 10 years is not untypical. Sometimes the sealing fails and water gets into the silicon, causing an early drop in output. Keit 121.215.141.120 (talk) 05:50, 10 February 2013 (UTC)
- In addition to Keit's excellent answer above, if you need to replace your batteries, you should be aware that the batteries sold for vehicle use are usually not suitable for long-term use with an inverter because they are not designed for deep discharge. Marine type batteries, or ones designed for your specific application should last much longer, but will cost at least twice as much (in the UK, at least). Dbfirs 08:40, 10 February 2013 (UTC)
- IIRC, the "never add water" batteries which have become ubiquitous for automobiles these days (contain calcium, I believe) are much more susceptible to damage by deep discharge than the old "check and fill periodically" variety; my personal experience is that one instance of leaving the headlights on overnight or similar can make them essentially "dead", i.e. they may work under ideal circumstances but leave them for a couple of weeks, or the first cold day, and no juice. So like the guy says, don't use car batteries. Gzuckier (talk) 05:22, 11 February 2013 (UTC)
- In addition to Keit's excellent answer above, if you need to replace your batteries, you should be aware that the batteries sold for vehicle use are usually not suitable for long-term use with an inverter because they are not designed for deep discharge. Marine type batteries, or ones designed for your specific application should last much longer, but will cost at least twice as much (in the UK, at least). Dbfirs 08:40, 10 February 2013 (UTC)
Do photons produce gravitational field ?
[edit]I know photons produce electric as well as magnetic field. I want to know, do they produce gravitational field ? I know electric field is always accompanied by magnetic field. Is it correct to say that "magnetic field is also always accompanied by electric field" ? There is another confusion, suppose if we increase the frequency of a photon, its energy also increases. If we increase the wavelength of a photon, its energy decreases. Since, E = hv. Give your response about last three sentences. Thanks! Parimal Kumar Singh (talk) 04:07, 10 February 2013 (UTC)
- Photons certainly interact with gravitational fields, gravitational lensing, for example. Also, not every electric field necessarily produces a magnetic field. A magnetic field is generated by a moving electric charge. A stationary electric charge produces an electric field, but no magnetic field. I'm not sure I follow your confusion on your last three sentences. Frequency and wavelength are inversely proportional: if you get more vibrations, the space between them gets shorter; if you get less vibrations, the space between them is longer. So decreasing energy = decreasing frequency = increasing wavelength and vice-versa. --Jayron32 05:05, 10 February 2013 (UTC)
- If an electric field is static, i.e., not moving or changing in strength, there is no resultant magnetic field as Jayron said. If the electric field is not static, there still will not be a magnetic field unless the field encloses at least part of an electric circuit - the magnetic field arises from the current in the circuit. Conversely, a magnetic field will not create an electric field if the space is a perfect electric conductor. In electromagnetic radiation, electric and magnetic fields propagate together. Wickwack 124.182.176.205 (talk) 05:36, 10 February 2013 (UTC)
- Photons contribute to the energy density of a volume, which could be included in a generalized energy tensor used to calculate behavior using general relativity. In most scenarios, the effect is negligible because the energy-density of photons has a much smaller magnitude than the mass density of ordinary matter. Nimur (talk) 06:07, 10 February 2013 (UTC)
When I was reading about Albert Einstein, I found that photons have attraction towards gravitational field. Earth has mass and it produces gravitational field. Do photons produce gravitational field like our earth? Parimal Kumar Singh (talk) 10:54, 10 February 2013 (UTC)
- Photons are massless, so they don't produce gravitational fields.Dja1979 (talk) 12:00, 10 February 2013 (UTC)
- See Stress–energy tensor. Radiation can indeed be a source of gravitational fields in General Relativity. Jheald (talk) 12:20, 10 February 2013 (UTC)
- They do produce a gravitational field due to the energy they have which depends on their frequency. If you had a box with mirrors it would weigh more if it had some light being reflected around inside it. Dmcq (talk) 12:25, 10 February 2013 (UTC)
- To elaborate a bit on the above answers, note that one can always choose a reference frame such that an electron's magnetic field "disappears". It still exists however in other reference frames. Furthermore, the electric and magnetic fields are mediated by the energy of photons, the precise value of which is frame dependent in accordance with E = hv and the relativistic Doppler effect. Also important is the fact that mass and energy are equivalent and since gravity is a consequence of mass-energy, light produces gravity and it is also affected by it. -Modocc (talk) 14:41, 10 February 2013 (UTC)
This is a hugely perennial question at the Refdesk and just from the first 20 search results for "photon" and "gravity" I find [1] [2] [3] (also [4] which is useless). Of these, [5] appears to be the useful answer if you can access it. At some point it would be great if someone could write an actual article about photon gravity explaining this and any other good sources so we can send people there. There's some unexpected factor of 2 involved. Wnt (talk) 16:03, 11 February 2013 (UTC)
- It's probably related to the factor-of-2 difference in the deflection of light by the sun, which is one of the most famous tests of general relativity. If so, it's more or less because spatial and temporal curvature contribute equally to the deflection when light is involved, while with nonrelativistic particles (and in the Newtonian limit) only the temporal curvature matters. I think. -- BenRG (talk) 19:25, 11 February 2013 (UTC)
Dumbed down Kindergarten level answer: Given that light is deflected by massive bodies, and given that photons have momentum, it follows from conservation of momentum that photons produce a gravitational field. Count Iblis (talk) 17:47, 12 February 2013 (UTC)
- Dumbed down high school level answer, at the least. The average kindergartener is gonna give you a "huh?" when you're talking about "photons", "conservation of momentum", and "gravitational fields". So will many, perhaps most, high schoolers. —SeekingAnswers (reply) 12:48, 14 February 2013 (UTC)
Planets and frequency of sound
[edit]Dear Sir, this is not my homework
- 1) Which planet of solar system-
- a) rotate faster
- b) rotate slower
- c) revolve around sun faster
- d) revolve around sun slowerthan any other planet?
- a) rotate faster
- 2) If i hear a sound of frequency 19,000 Hz, will it cause pain to my ears? What is relation between frequency and decibel?
Thank you. Walker — Preceding unsigned comment added by C. Walker19 (talk • contribs) 07:22, 10 February 2013 (UTC)
- You will find the answers to 1) in the article Planet#Planetary_attributes. Please let us know if you need help interpreting this. Frequency is the pitch of a note, and 19 kHz is at the top of the hearing range, in fact most of the population cannot hear a note this high. If you are young, then maybe you can, especially if it is loud. Decibels are a measure of sound intensity or loudness. See the article Decibel for technical details. Any very loud sound can be perceived as pain and may cause hearing damage. Dbfirs 08:27, 10 February 2013 (UTC)
- Agreed, and let me add one more tidbit. It takes less energy to produce high frequency sounds of a given volume than low frequency sounds of the same volume. Therefore, say 20 watts at 19 kHz may very well be painful, while 20 watts at at a lower frequency is not. StuRat (talk) 03:57, 11 February 2013 (UTC)
Tensor
[edit]I was searching for a simple definition of tensor, I tried to read the linked article, but it was beyond my knowledge. Then, a professor told me a simple definition - A physical quantity is said to be tensor if it is neither a scalar nor a vector as its direction is not properly specified, but has different values in different directions. Examples of tensor include strain, moment of inertia, density, refractive index, etc. Wikipedia article on tensor says just opposite - Vectors and scalars themselves are also tensors. Who is correct? Which one should I prefer? Sunny Singh 07:57, 10 February 2013 (UTC) — Preceding unsigned comment added by Sunnysinghthebaba (talk • contribs)
- Oh, you've stepped in it now :-)
- The simple answer to your question is that scalars and vectors are tensors, of rank 0 and 1 respectively, but you don't normally call them that, because why bother? Your professor was explaining tensors of rank 2 and beyond. --Trovatore (talk) 08:00, 10 February 2013 (UTC)
- You might enjoy Dan Fleisch's "What's a Tensor?" video in which he uses "children's blocks, small arrows, a couple of pieces of cardboard and a pointed stick".[6] Sean.hoyland - talk 13:54, 10 February 2013 (UTC)
- I'm biased due to my computer science background, but I think the simplest way to think about a tensor is as a multidimensional array. Imagine a one-dimensional array, say arr[32]. In mathematics, this represents a vector, which is a rank-1 tensor. Imagine a two-dimensional array, arr[32][32]. This is a rank 2 tensor, and every value arr[i][j] presumably has some physical meaning specific to the indices i and j. Similarly, arr[32][32][32] would be a 3-dimensional array and hence a rank-3 tensor, etc.
- Here's a concrete example to help with your intuition. The stress-energy tensor describes the movement of four-momentum through spacetime. It's a rank 2 tensor, so you can imagine it as the array T[4][4]; it's 4x4 because spacetime has 4 dimensions. T[i][j], the jth column of the ith row of the array, is the flux of the ith component of momentum through the jth dimension. For example, since by convention the 1st dimension is x, T[1][1] is the flux of the momentum in the x direction, across the surface defined by constant x. Similarly, T[1][2] is the flux of the momentum in the x direction, across the surface defined by constant y. --140.180.243.51 (talk) 19:38, 10 February 2013 (UTC)
- The problem with that formulation is that it's coordinate-specific (or basis-specific, depending on whether you're thinking of physics-style or algebra-style tensors).
- To me a rank-two tensor is a machine that takes one vector and gives you back another. For the stress tensor, you can think about the input vector being the one that describes a little surface of a place you could potentially put a cut inside the object, and the output vector is how much pressure or shear the object puts on that surface. None of this needs any coordinate system to be specified, and you don't need to break it down in to components to understand it. (When you want to make a practical calculation, you probably will specify a coordinate system and take components, but you'll use whichever one is most convenient at the time rather than one specified in advance.) --Trovatore (talk) 23:50, 10 February 2013 (UTC)
Hydrogen Synthesization
[edit]Can hydrogen be synthesized by manipulation of subatomic particles? — Preceding unsigned comment added by Lawrie1 (talk • contribs) 13:56, 10 February 2013 (UTC)
- Sure. Take one proton and one electron, bring them into contact: presto, you have a hydrogen atom. Looie496 (talk) 16:17, 10 February 2013 (UTC)
- All of the science of chemistry could be described as "manipulation of subatomic particles" - but as Looie says, we can also do it directly from a stream of protons and a stream of electrons. SteveBaker (talk) 15:53, 11 February 2013 (UTC)
Power lines: buried or overground?
[edit]What are the advantages of one system or the other? Is it just the cost of implementing vs. the cost of maintaining? OsmanRF34 (talk) 16:06, 10 February 2013 (UTC)
- It depends on how you define "advantages". Ruslik_Zero 16:47, 10 February 2013 (UTC)
- "Underground cables take up less right-of-way than overhead lines, have lower visibility, and are less affected by bad weather. However, costs of insulated cable and excavation are much higher than overhead construction. Faults in buried transmission lines take longer to locate and repair. Underground lines are strictly limited by their thermal capacity, which permits less overload or re-rating than overhead lines. Long underground cables have significant capacitance, which may reduce their ability to provide useful power to loads." See our article Electric power transmission, also Overhead power line and Undergrounding. Alansplodge (talk) 16:52, 10 February 2013 (UTC)
- There are also safety considerations that generally favor underground lines. Looie496 (talk) 16:57, 10 February 2013 (UTC)
- Also note that there are two variants of underground power lines, those with access tunnels and those without. Most seem to lack access tunnels, and even go under streets, etc., requiring digging up those streets to access them. Those with access tunnels, while more expensive initially, make for far easier, quicker, and cheaper maintenance, upgrades, etc.
- And, in the comparison of above ground to underground, we can't neglect that most people find above ground wires to be ugly. As such, they may bring down property values. StuRat (talk) 04:03, 11 February 2013 (UTC)
- True, but the higher construction and maintenance costs of underground cables still appear to trump everything else. 24.23.196.85 (talk) 04:11, 11 February 2013 (UTC)
- That depends very much on local conditions. In Germany, overland lines are above ground (as mentioned before, if you distribute AC, underground has significantly larger losses over long distance), but local distribution is nearly exclusively underground. It must be 30 years or so since I last saw an overhead line go into a private building. The cost advantage shifts based on population density, and also on Quality of Service. --Stephan Schulz (talk) 12:48, 11 February 2013 (UTC)
Breakdown of a Barrel of Oil
[edit]Oil is used to produce fuels and petrochemicals. I believe that more of the oil is used for petrochemicals and for fuels, and that an oil company's profits are derived more from petrochemicals than from fuels. Is there an entry that provides the (approximate) breakdown? 68.54.32.39 (talk) 17:39, 10 February 2013 (UTC)
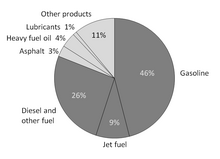
- Crude oil is what's taken from the ground and comes in barrels; it's described as sweet if it has low sulfur content which makes it easier to work with. The article gives average contents. It is then refined by heating and distillation and various other chemical processes that separate out and create different petrochemicals. So it's not so much that they just separate out what's already there as it is what they make from it. μηδείς (talk) 18:03, 10 February 2013 (UTC)
- Most refineries use distillation to separate the crude oil into different length hydrocarbons. They also use fluid catalytic cracking to split long-chain molecules into more-in-demand shorter ones. It is also possible to merge short-chained hydrocarbons into longer ones, which might be useful if there is more methane that there is a demand for. CS Miller (talk) 19:44, 10 February 2013 (UTC)
- Does this chart work for you? I found it at petroleum product. --Jayron32 20:07, 10 February 2013 (UTC)
- I've added the article caption to the chart to make clear that the product listed are not the unrefined makeup of a barrel. I'm also moving this up to my post to save space--if you object, go ahead and revert the edit. μηδείς (talk) 03:54, 11 February 2013 (UTC)
- Thanks for that by the way. Good call. --Jayron32 04:05, 11 February 2013 (UTC)
- What are those percentages though? Percent by volume? By weight? By energy content? Either way, that wouldn't answer the OP's question which is "By dollar value". The original WP:RS upon which that chart is based [7] is similarly unclear. Any chart with unlabelled axes is essentially useless!! SteveBaker (talk) 15:44, 11 February 2013 (UTC)
- Not to mention, that EIA chart indicates refinery output, not refinery output per barrel of oil. A significant amount of refined product is re-run through the refinery; so for example, if your refinery starts with one barrel of crude, you might produce twenty gallons valuable gasoline and twenty gallons of low-value heavy asphaltenes; so you sell what you can and dump the "waste" asphalt back in with the next barrel of crude. From that, you might produce twenty-two gallons of gasoline and ... twenty more gallons of asphalt and miscellaneous products. (Hopefully not in those ratios!) The real world of refinery chemistry is not as straightforward as a pie-chart makes it look! Here's my favorite refinery poster (the one I used to hang on my wall for reference): Mustang Engineering's Modern Refinery, one of many excellent promotional posters they produce. (Though, the PDF is no longer available online, you can order a copy from them). Nimur (talk) 05:47, 12 February 2013 (UTC)
- What are those percentages though? Percent by volume? By weight? By energy content? Either way, that wouldn't answer the OP's question which is "By dollar value". The original WP:RS upon which that chart is based [7] is similarly unclear. Any chart with unlabelled axes is essentially useless!! SteveBaker (talk) 15:44, 11 February 2013 (UTC)
- Thanks for that by the way. Good call. --Jayron32 04:05, 11 February 2013 (UTC)
- I've added the article caption to the chart to make clear that the product listed are not the unrefined makeup of a barrel. I'm also moving this up to my post to save space--if you object, go ahead and revert the edit. μηδείς (talk) 03:54, 11 February 2013 (UTC)
<-I'm not sure the premise of this question is entirely valid. It's probably fair to say that to a first approximation, oil company income comes from finding and producing hydrocarbons (i.e. their "upstream" actvities) rather than doing things to those hydrocarbons (i.e. their "downstream" activities). Obviously it's a lot more complicated than that in reality and each company is different in terms of the upstream vs downstream activities/profitability, but Exxon Mobil earnings for 2011 after tax for example were ~41 billion dollars, ~34 of which came from their upstream operations. Sean.hoyland - talk 04:27, 11 February 2013 (UTC) As original poster of the question, I thank y'all for the inputs. Right, a simple question but the answer is complex. 68.54.32.39 (talk) 20:41, 12 February 2013 (UTC)
Upright exercise bike question
[edit]What should be the position of the knees relative to the feet while riding an upright stationary bike? I mean should the knees be in the same level of the feet (distance between feet = distance between knees), or the knees be slightly bent outwards (distance between feet < distance between knees)? Which is the correct posture to prevent knee injury? Is 18 inches distance between the outer edges of the the two feet when they the over the pedals a safe distance? --PlanetEditor (talk) 17:47, 10 February 2013 (UTC)
- The knees should be in the same plane as the feet, as nearly as possible. I can't speak to specific distances, but generally you want your knee bending in the way it naturally bends, not in any other direction. Riding significantly bowlegged is bad. Looie496 (talk) 19:44, 10 February 2013 (UTC)
- Thanks. --PlanetEditor (talk) 03:01, 11 February 2013 (UTC)
Asteroid 2012 DA14 and orbital resonance
[edit]After asteroid 2012 DA14 passes by the Earth in a few days, its orbital period will be 317 days. That is very close to a 7:6 ratio with Earth's orbital period. Is this significant - i.e. an orbital resonance? Bubba73 You talkin' to me? 19:39, 10 February 2013 (UTC)
- A resonance is a result of many small perturbations accumulating to give a stable relationship. In this case the 317 day period will result from a single huge perturbation, the previous period being 368 days, so it couldn't possibly be a resonance. Generally speaking Earth's gravity is too weak in comparison to Jupiter's for Earth to be able to produce stable orbital resonances. Looie496 (talk) 19:51, 10 February 2013 (UTC)
- Thank you Bubba73 You talkin' to me? 20:27, 10 February 2013 (UTC)
 Resolved
Resolved - The exception being resonances with very low denominators - particularly 1:1 resonances. See horseshoe orbit for instance. --Tango (talk) 19:00, 11 February 2013 (UTC)
- 99942 Apophis is affected by the Earth, isn't it? Bubba73 You talkin' to me? 00:39, 12 February 2013 (UTC)
- Certainly, but not to the extent of being in resonance. Everything in the (observable) universe is affected by everything else to some extent. --Tango (talk) 12:27, 12 February 2013 (UTC)
- 99942 Apophis is affected by the Earth, isn't it? Bubba73 You talkin' to me? 00:39, 12 February 2013 (UTC)
- Thank you
- Whoops, I actually meant 3753 Cruithne. Bubba73 You talkin' to me? 01:52, 13 February 2013 (UTC)
Dr. Imenson (spelling?) medical doctor
[edit]Dr. Imeson (not sure of the spelling) was my family doctor in the late 1940's and into the 1950's. I would like any information on him. What happened to him. When he passed on, etc.. — Preceding unsigned comment added by 24.7.138.9 (talk • contribs) 17:25, February 10, 2013
I need to add that he was a doctor in San Francisco, California — Preceding unsigned comment added by 24.7.138.9 (talk • contribs) 17:28, February 10, 2013
- There are plenty of people-search websites you could try. A normal Google search turns up the following possibility:
- Dr. Shale Imeson, MD Anesthesiologist, Pain Management Physician in Stockton, CA. However, since he's still around, it is unlikely that he was a Dr. in the '40s-'50s -- Maybe his father? ~:74.60.29.141 (talk) 23:46, 10 February 2013 (UTC)
