User talk:Professor Polt
Please add your contributions to "methods for the synthesis of alpha-D-ethyl-glucopyranoside" or "methods for the synthesis of beta-D-ethyl-glucopyranoside" on this page. Please do not edit anyone else's addition. If you are not in my class, please don't post anything here.
Kevin A. Scott — Preceding unsigned comment added by Sculpturatus (talk • contribs) 23:52, 21 April 2016 (UTC)
- REDIRECT [[1]]
Various syntheses of ethylglucopyranose are described, including enzymatic and non-enzymatic methods, as well as protected and unprotected sugars used as starting materials. — Preceding unsigned comment added by Sculpturatus (talk • contribs) 23:51, 21 April 2016 (UTC)
Aellsworth13 (talk) 16:48, 20 April 2016 (UTC) Dipak K. Roy & Manobjyoti Bordoloi Montmorillonite K-10 as a Reusable Catalyst for Fischer Type of Glycosylation under Microwave Irradiation Journal of Carbohydrate Chemistry, 27:5, 300-307, 2008, DOI: 10.1080/07328300802107437

— Preceding unsigned comment added by Aellsworth13 (talk • contribs) 00:55, 8 May 2016 (UTC)
HAVE THE REST DONE, HAVING TROUBLE UPLOADING IT
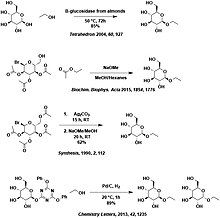
Pedro M. L. Goncalves, Stanley M. Roberts, and Peter W. H. Wan Regioselective acylation of carbohydrate derivatives using lipases leading to a facile two-step procedure for the separation of some a- and b-glucopyranosides and galactopyranosides Tetrahedron 60 (2004) 927–932
HAVE THE REST DONE, HAVING TROUBLE UPLOADING IT
Wei Li, Kazuo Koike, Yoshihisa Asada, Takafumi Yoshikawa, Tamotsu Nikaido, Biotransformation of low-molecular-weight alcohols by Coleus forskohlii hairy root cultures Carbohydrate Research 338, (2003), 729-731
HAVE THE REST DONE, HAVING TROUBLE UPLOADING IT Aellsworth13 (talk) 16:48, 20 April 2016 (UTC)Alyssa Ellsworth
Steven Petritis:
1) Authors: Wen-Ya Lu, Guo-Qiang Lin, Hui-Lei Yu, Ai-Ming Tong, and Jian-He Xu Title: "Facile synthesis of alkyl beta-D-glucopyranosides from D-glucose and the corresponding alcohols using fruit seed meals" Journal: Journal of Molecular Catalysis B: Enzymatic Year: 2007 Volume: 44 Page Numbers: 72-77 Reference: W.-L. Lu et al. Journal of Molecular Catalysis B: Enzymatic 2007, 44, 72-77. Description: The authors present a cheap and synthetically facile biotransformation of D-glucose to anomerically pure alkyl beta-D-glucopyranosides. The use of commercially available fruit seed meals allows this enzymatic method to be done in one step under mild conditions, to be highly selective for beta alkyl D-glucopyranoside products, and to be conducted using completely unprotected sugar starting materials.

2) Authors: Wang Qinqqin, Yu Huilei, Zhao Na, Li Chunxiu, Shang Yazhuo, Liu Honglai, Xu Jianhe Title: "Significantly Improved Equilibrium Yield of Long-Chain Alkyl Glucosides via Reverse Hydrolysis in a Water-Poor System Using Cross-Linked Almond Meal as a Cheap and Robust Biocatalyst" Journal: Chinese Journal of Catalysis Year: 2012 Volume: 33 Page Numbers: 275-280 Reference: W. Qinqqin et al. Chinese Journal of Catalysis 2012, 33, 275-280. Description: The authors developed two new methods for the enzymatic synthesis of a wide array of beta-D-glucopyranosides that exhibit exclusive beta selectivity at the anomeric carbon. These two new approaches are reverse hydrolysis (a thermodynamically-driven method) and transglycosation (a kinetically-driven method). Additionally, the equilibrium and Gibbs' Free Energy of these glycosidic reactions were studied for the first time.
3) Authors: Matthias Nüchter, Bernd Ondruschka, and Werner Lautenschläger Title: "Microwave-Assisted Synthesis of Alkyl Glycosides" Journal: Synthetic Communications Year: 2001 Volume: 31 (9) Page Numbers: 1277-1283 Reference: M. Nüchter et al. Synthetic Communications 2001, 31 (9), 1277-1283. Description: The authors of this paper suggest a new microwave-assisted methodology for the synthesis of commonly made alpha and beta alkyl glycosides. The advantage of this new process is that reaction times can be reduced from between 48-72 hours to 30 minutes or less depending on the alkyl glycoside of interest. Although complete selectivity for either the alpha or beta product was not achieved, due to competition with the Koenigs-Knorr synthesis, this new method has been shown to be substantially more economically, ecologically, and synthetically advantageous than other current synthetic routes.
Ollie Snell
Solid acid catalysed formation of ethyl levulinate and ethyl glucopyranoside from mono- and disaccharides Shunmugavel Saravanamurugan, Anders Riisager ⁎ Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark
The method of formation of beta-D-ethyl-glucopyranoside proposed by Saravanamurugan et al. involves the use of the sulfonic acid functionalised SBA-15 catalyst, SO3H-SBA-15-D. With glucose as a starting material, addition of ethanol over the catalyst produced beta-D-ethyl-glucopyranoside in an 80% yield. This is an improved yield compared to using zeolite catalysts, due to problems with their small pore openings (1). Limitations of this method include the fact that on increasing the glucose concentration by 3, the yield dropped to 72%. The yields also varied for different sugar starting materials. Using cellobiose for example, a disaccharide consisting of two glucose units, resulted in a lower yield of 66%. Moreover, using sucrose resulted in formation of two products due to its breakdown into glucose and fructose monosaccharides. Fructose went on to form hydroxymethylfurfural and ultimately ethyl levulinate (27% yield), meaning the yield for the formation of beta-D-ethyl-glucopyranoside was significantly lower at 35%. However, the catalyst shows a great effectiveness in conversion of glucose and other sugars to beta-ethyl-glucopyranoside compared to zeolites so this method can prove very useful in catalytic organic synthesis.
(1) J.W. Park, J.H. Kim, G. Seo, Polymer Degradation and Stability 76 (2002) 495–501 — Preceding unsigned comment added by Pcyos2 (talk • contribs) 03:02, 22 April 2016 (UTC) Pcyos2 (talk) 03:37, 22 April 2016 (UTC)
A convenient stereoselective synthesis of β-D-glucopyranosides, Vishal Y Joshi et al., Indian journal of chemistry, Vol. 45B, February 2006, pp. 461-465.
Joshi et at. propose the Koenigs-Knorr method in the stereoselective synthesis of alkyl D-glucopyranosides via glycosylation, with the exception of using lithium carbonate which is less expensive and toxic than the conventional method of using silver or mercury salts. D-glucose is first protected by forming the peracetate by addition of acetic anhydride in acetic acid, and then addition of hydrogen bromide which brominates at the 5-position. On addition of the alcohol ROH and lithium carbonate, the OR replaces the bromine and on deprotecting the acetylated hydroxyls the product is produced in relatively high purity. It was suggested by Joshi et al. that lithium acts as the nucleophile that attacks the carbon at the 5-position and through a transition state the alcohol is substituted for the bromine group. Advantages of this method as well as its stereoselectivity and low cost of the lithium salt include that it can be done at room temperature and its yield compares relatively well with the conventional Koenigs-Knorr method (1).
(1) Koenigs W & Knorr E, Chem Ber, 34,1901, 957.

Pcyos2 (talk) 04:24, 22 April 2016 (UTC)
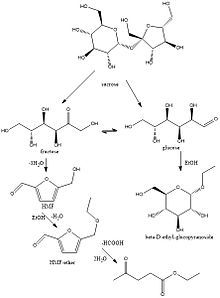
One-pot conversion of carbohydrates into 5-ethoxymethylfurfural and ethyl D-glucopyranoside in ethanol catalyzed by a silica supported sulfonic acid catalyst, Bing Liu and Zehui Zhang, RSC Advances, 2013, 3, 12313.
Liu and Zhang propose a high yielding preparation of ethyl D-glucopyranosides from glucose and ethanol using a silica supported sulfonic acid catalyst. Although the reaction gives a 91.7% yield, the product formed is a racemic mixture of the alpha and beta product and so the reaction is only useful if there is no desire for stereoselectivity. However, as well as a high yield and the relatively cheap catalyst, this method can prove useful for the use of ethyl D-glucopyranosides in the surfactant industries as well as for chemical intermediates in other synthetic processes (1). AND it’s British
(1) D. Z. Wei, P. Zou, M. B. Tu and H. Zheng, J. Mol. Catal. B: Enzym., 2002, 18, 273.
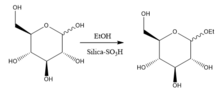
Pcyos2 (talk) 04:48, 22 April 2016 (UTC)
Foley
[edit]1) Protection-free Synthesis of Alkyl Glycosides under Hydrogenolytic Conditions Ishihara, Masaki; Takagi, Yuka; Li, Gefei; Noguchi, Masato; Shoda, Shin-Ichiro Chemistry Letters, 2013 , vol. 42, # 10 p. 1235 - 1237
2) Enzyme Catalysed Preparation of 6-O-Acylglucopyranosides 6-O-Monoesters of alkyl glucopyranosides have been prepared on a large scale in a more than 90% yield by direct enzyme-catalysed esterification of glucopyranosides with long chain fatty acids in a solvent-free process. Synthesis 1990; 1990(2): 112-115 DOI: 10.1055/s-1990-26802 — Preceding unsigned comment added by MCRsForDayz (talk • contribs) 17:25, 25 April 2016 (UTC)
3)
Hg(NO3)2 in acetonitrile
Chemistry of the glycoside linkage. Exceptionally fast efficient formation of glycosides by remote activation Hanessian, Stephen; Bacquet, Christian; Lehong, Nghiep Carbohydrate Research, 1980 , vol. 80, p. C17 - C22
4) Product distribution
2,6-dimethylpyridine T=100°C
Sinnott, Michael L.; Jencks, William P. Journal of the American Chemical Society, 1980 , vol. 102, # 6 p. 2026 - 2032
5) With Dowex-50 H+ 20 h; EtOH Bisht; Gross; Cholli Applied Spectroscopy, 1998 , vol. 52, # 11 p. 1472 - 1478 — Preceding unsigned comment added by MCRsForDayz (talk • contribs) 05:06, 22 April 2016 (UTC)
1) G. Vic and D. Thomas, Tetrahedron Letters 33, 4567 (1992) 2) M. Nüchter, B. Ondruschka, and W. Lautenschläger, Synthetic Communications 31, 1277 (2001) 3) G. R. Vijayakumar, C. George, and S. Divakar, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry 46, 314 (2007) — Preceding unsigned comment added by Yegor gt (talk • contribs) 00:20, 25 April 2016 (UTC)
Yegor
[edit]1) G. Vic and D. Thomas, Tetrahedron Letters 33, 4567 (1992)
A useful method for enzyme catalyzed stereoselective glucosilation in organic media. A wide range of alcohols can be used. Protection steps are not necessary.
2) M. Nüchter, B. Ondruschka, and W. Lautenschläger, Synthetic Communications 31, 1277 (2001)
This method produces 100% yield of α- and β-D-glucosides with the ability to be performed on a multi-kilogram scale.
3) G. R. Vijayakumar, C. George, and S. Divakar, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry 46, 314 (2007)
A method for glucosilation using a commercially available enzyme amyloglucosidase. This method produces higher yields with higher molecular weight alcohols and is pH sensitive. — Preceding unsigned comment added by Yegor gt (talk • contribs) 01:21, 25 April 2016 (UTC)
Laura
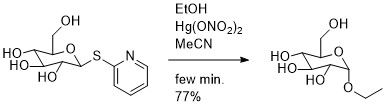
[edit]1)Ferrier, R. J.; Hay, R. W.; Vethaviyasar, N. Carbohydr. Res. 1973, 27, 55.
The reaction of 2-pyridylthio β-D-glucopyranoside in the presence of an alcohol and mercuric nitrate in acetonitrile does yield the desired α-D-glucopyranoside but only in a modest 2:1 ratio of α:β.
2) Hanessian, S.; Lou, B. Chem. Rev. 2000, 100, 4443
Inspired by the work done by Ferrier et. al. Hanessian improved the α:β ratio by changing the leaving group to a methoxy pyridyloxy (MOP) leaving group that increased the α:β to 10:1 when MeOTF and a 1:1 mixture of nitromethane and the desired alcohol were used instead. — Preceding unsigned comment added by Dinosaurs.907 (talk • contribs) 05:54, 26 April 2016 (UTC)
Ali
[edit]1) Chemoenzymatic Synthesis of b-d-Glucosides using Cellobiose Phosphorylase from Clostridium thermocellum, K.D. Winter, L.V. Renterghem, K. Wuyts, H. Pelantov, V. Krˇen, W. Soetaert, T. Desmeta; Adv. Synth. Catal. 2015, 357, 1961 –1969.
2) Convenient synthesis of alkyl and phenylalkyl beta-d-glucopyranosides using facile and novel biocatalysts of plant origin, R. Yanga, Z. Wanga, Y. Bia, J. Jia, X. Zhaoa, X. Liub, W. Dua; Industrial Crops and Products 74 (2015) 918–924.
3) Sulfuric acid immobilized on silica: an excellent catalyst for Fischer type glycosylation, B. Roy, B. Mukhopadhyay; Tetrahedron Letters 48 (2007) 3783–3787. — Preceding unsigned comment added by AANaeini (talk • contribs) 22:49, 5 May 2016 (UTC)
