User:QuantumProtein/Cyclic guanosine monophosphate
All changes for cGMP will be in this sandbox.
Here are a list of sources I am planning on using
Review Articles:
https://pmc.ncbi.nlm.nih.gov/articles/PMC3263465/
https://pmc.ncbi.nlm.nih.gov/articles/PMC1369255/
https://pmc.ncbi.nlm.nih.gov/articles/PMC3932363/
Primary Article:
https://www.jacc.org/doi/full/10.1016/j.jacc.2020.08.031[1]
https://www.ahajournals.org/doi/full/10.1161/JAHA.119.013966[2]
https://pmc.ncbi.nlm.nih.gov/articles/PMC8090939/[3]
Image:
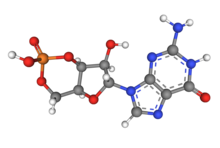
I want to change the current image that uses the space filling model to a ball and stick, this will allow for someone to better see the structure.
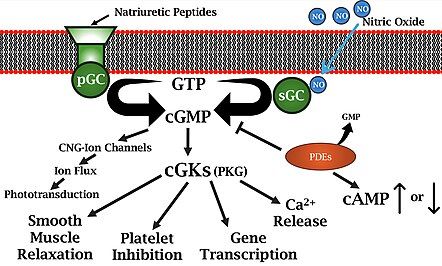
Also create a better version of cGMP protein pathway Map that better explains it with respect to physiology.
I want to clean up the introduction paragraph and remove the last sentence as it an odd addition to the overview.
History
[edit]Cyclic guanosine monophosphate (cGMP) research began after cGMP and cyclic adenosine monophosphate (cAMP) were identified as cellular components and potentially involved with cellular regulation.[4] Upon the synthesis of cGMP in 1960,[4] progress rapidly spread in the understanding of regulation and effects of cGMP. Earl W. Sutherland received the 1971 Nobel Prize in Medicine for his work with cAMP and secondary messengers. This award sparked extensive research into cAMP, while cGMP received less attention, with its biological functions largely unknown until the 1980s.[5] During this period, two pivotal discoveries highlighted cGMP’s role in cellular signaling: atrial natriuretic peptide (ANP) was found to stimulate cGMP synthesis through the particulate guanylyl cyclase (pGC) receptor, and nitric oxide (NO), identified as the endothelium-derived relaxing factor, was shown to activate soluble guanylyl cyclase (sGC), producing cGMP to mediate vasodilation in smooth muscle cells.[5] Further components involved with the cGMP were also identified such as cGMP-hydrolyzing phosphodiesterases (PDEs) and cGMP-binding proteins.[5] The awarding of the 1998 Nobel Prize to Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad for their discoveries in the NO-cGMP pathway renewed interest in cGMP research with the 1st International Conference on cGMP being held in 2003.[5]
Functions
[edit]cGMP acts as a regulator of ion channel conductance, glycogenolysis, cellular apoptosis, and platelet inhibition. cGMP relaxes smooth muscle tissue leading to vasodilation which increases blood flow. Additionally, cGMP is involved with neurogenesis and neuroplasticity. ADD last sentence of current wiki
Change SGC to sGC
Pathology
[edit]Role in Cardiovascular Events
[edit]The nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-phosphodiesterase (PDE) pathway has become a target in developing treatments for heart failure. A deficit in cGMP levels has been associated with adverse cardiovascular outcomes, promoting factors like myocardial fibrosis, vasoconstriction, and inflammation, all of which accelerate heart failure progression.[1] Some soluble guanylate cyclase (sGC) stimulators, have yielded promising outcomes in reducing cardiovascular events. [1] Their effectiveness is thought to result from increased sensitivity of sGC to endogenous NO.
Elevated plasma cGMP levels, regulated predominantly by natriuretic peptides (NP) rather than nitric oxide (NO), were found to correlate with a higher risk of heart failure, atherosclerotic cardiovascular disease, and coronary heart disease. [2]
Role in Major Depression Disorder
[edit]The cGMP signaling pathway plays a role in the regulation of neuroplasticity, an area of interest in understanding the pathophysiology of major depressive disorder (MDD).[6] The cGMP signaling pathway in the brain operates as a second messenger system, amplifying neurotransmitter signals, influencing gene expression and neuronal function. Within neurons, cGMP levels are modulated by guanylate cyclase enzymes, which synthesize cGMP, and by PDEs, which degrade cGMP.[6]
Enhancing cGMP levels, either by stimulating guanylate cyclase or inhibiting PDEs, promotes neurogenesis and synaptic plasticity, particularly in brain regions implicated in MDD, such as the hippocampus and prefrontal cortex.[6] Animal studies also demonstrate that chronic antidepressant treatment can elevate cGMP levels in these areas, suggesting a possible link between increased cGMP activity and antidepressant efficacy.[6] Genetic research has further highlighted specific polymorphisms in PDE genes associated with MDD susceptibility and treatment response.[6]
Role in Infectious Disease Pathogenesis
[edit]Certain pathogens, such as Enterotoxigenic Escherichia coli (ETEC), elevate cGMP to evade host immune defenses and establish infection. ETEC’s heat-stable toxin induces significant cGMP production within intestinal epithelial cells, and this cGMP is often secreted into the extracellular space, where it serves as a signaling molecule.[3] Extracellular cGMP, in turn, triggers the release of IL-33 release which modulate inflammation and impact the immune system’s ability to mount effective responses, dampening both innate and adaptive immunity. [3][7]
Protein kinase activation
[edit]The cGMP-dependent protein kinase (PKG) activation pathway begins with the production of cGMP by guanylyl cyclase enzymes, which can be activated by signaling molecules such as nitric oxide (NO) or natriuretic peptides. Elevated cGMP levels then lead to the activation of some protein-dependent kinases like PKG.[5]
(Back to wiki paragraph)
Once activated, PKG phosphorylates various target proteins, altering their function and contributing to cellular processes such as smooth muscle relaxation, ion channel regulation, and inhibition of platelet aggregation.This pathway is also significant in cardiovascular physiology, where it helps maintain vascular tone and blood pressure.[1]
See also
[edit]References
[edit]- ^ a b c d Emdin, Michele; Aimo, Alberto; Castiglione, Vincenzo; Vergaro, Giuseppe; Georgiopoulos, Georgios; Saccaro, Luigi Francesco; Lombardi, Carlo Mario; Passino, Claudio; Cerbai, Elisabetta; Metra, Marco; Senni, Michele (2020-10-13). "Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week". Journal of the American College of Cardiology. 76 (15): 1795–1807. doi:10.1016/j.jacc.2020.08.031. ISSN 0735-1097 – via Elsevier Science Direct.
- ^ a b Zhao, Di; Guallar, Eliseo; Vaidya, Dhananjay; Ndumele, Chiadi E.; Ouyang, Pamela; Post, Wendy S.; Lima, Joao A.; Ying, Wendy; Kass, David A.; Hoogeveen, Ron C.; Shah, Sanjiv J.; Subramanya, Vinita; Michos, Erin D. (2020-01-21). "Cyclic Guanosine Monophosphate and Risk of Incident Heart Failure and Other Cardiovascular Events: the ARIC Study". Journal of the American Heart Association. 9 (2). doi:10.1161/JAHA.119.013966. ISSN 2047-9980.
- ^ a b c Motyka, Natalya I.; Stewart, Sydney R.; Hollifield, Ian E.; Kyllo, Thomas R.; Mansfield, Joshua A.; Norton, Elizabeth B.; Clements, John D.; Bitoun, Jacob P. (2021-03-17). Torres, Victor J. (ed.). "Elevated Extracellular cGMP Produced after Exposure to Enterotoxigenic Escherichia coli Heat-Stable Toxin Induces Epithelial IL-33 Release and Alters Intestinal Immunity". Infection and Immunity. 89 (4). doi:10.1128/IAI.00707-20. ISSN 0019-9567.
- ^ a b Kots, Alexander Y.; Martin, Emil; Sharina, Iraida G.; Murad, Ferid (2009), Schmidt, Harald H. H. W.; Hofmann, Franz; Stasch, Johannes-Peter (eds.), "A Short History of cGMP, Guanylyl Cyclases, and cGMP-Dependent Protein Kinases", cGMP: Generators, Effectors and Therapeutic Implications, vol. 191, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 1–14, doi:10.1007/978-3-540-68964-5_1, ISBN 978-3-540-68960-7, retrieved 2024-11-27
- ^ a b c d e Feil, Robert; Kemp‐Harper, Barbara (Feb 2006). "cGMP signalling: from bench to bedside: Conference on cGMP Generators, Effectors and Therapeutic Implications". EMBO reports. 7 (2): 149–153. doi:10.1038/sj.embor.7400627. ISSN 1469-221X.
- ^ a b c d e W. Reierson, Gillian; Guo, Shuyu; Mastronardi, Claudio; Licinio, Julio; Wong, Ma-Li (2011-12-01). "cGMP Signaling, Phosphodiesterases and Major Depressive Disorder". Current Neuropharmacology. 9 (4): 715–727. doi:10.2174/157015911798376271.
- ^ Wang, Haixiu; Zhong, Zifu; Luo, Yu; Cox, Eric; Devriendt, Bert (2019-01-08). "Heat-Stable Enterotoxins of Enterotoxigenic Escherichia coli and Their Impact on Host Immunity". Toxins. 11 (1): 24. doi:10.3390/toxins11010024. ISSN 2072-6651.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
