User:Professional Crastination/sandbox
Evidence of treatment efficacy
[edit]Binge Eating Disorder
[edit]Binge eating disorder (BED) is characterized by recurrent and persistent episodes of compulsive binge eating.[1] These episodes are often accompanied by marked distress and a feeling of loss of control over eating.[1] The pathophysiology of BED is not fully understood, but it is believed to involve dysfunctional dopaminergic reward circuitry along the cortico-striatal-thalamic-cortical loop.[2][3] As of July 2024, lisdexamfetamine is the only USFDA- and TGA-approved pharmacotherapy for BED.[4][5] Evidence suggests that lisdexamfetamine's treatment efficacy in BED is underpinned at least in part by a psychopathological overlap between BED and ADHD, with the latter conceptualized as a cognitive control disorder that also benefits from treatment with lisdexamfetamine.[2][3]

Lisdexamfetamine's therapeutic effects for BED primarily involve direct action in the central nervous system after conversion to its pharmacologically active metabolite, dextroamphetamine.[5] Centrally, dextroamphetamine increases neurotransmitter activity of dopamine and norepinephrine in prefrontal cortical regions that regulate cognitive control of behavior.[2][5] Similar to its therapeutic effect in ADHD, dextroamphetamine enhances cognitive control and may reduce impulsivity in patients with BED by enhancing the cognitive processes responsible for overriding prepotent feeding responses that precede binge eating episodes.[2][7][8] In addition, dextroamphetamine's actions outside of the central nervous system may also contribute to its treatment effects in BED. Peripherally, dextroamphetamine triggers lipolysis through noradrenergic signaling in adipose fat cells, leading to the release of triglycerides into blood plasma to be utilized as a fuel substrate.[3][9] Dextroamphetamine also activates TAAR1 in peripheral organs along the gastrointestinal tract that are involved in the regulation of food intake and body weight.[6] Together, these actions confer an anorexigenic effect that promotes satiety in response to feeding and may decrease binge eating as a secondary effect.[8][6]
Medical reviews of randomized controlled trials have demonstrated that lisdexamfetamine, at doses between 50-70 mg, is safe and effective for the treatment of moderate-to-severe BED in adults.[sources 1] These reviews suggest that lisdexamfetamine is persistently effective at treating BED and is associated with significant reductions in the number of binge eating days and binge eating episodes per week.[sources 1] Furthermore, a meta-analytic systematic review highlighted an open-label, 12-month extension safety and tolerability study that reported lisdexamfetamine remained effective at reducing the number of binge eating days for the duration of the study.[8] In addition, both a review and a meta-analytic systematic review found lisdexamfetamine to be superior to placebo in several secondary outcome measures, including persistent binge eating cessation, reduction of obsessive-compulsive related binge eating symptoms, reduction of body-weight, and reduction of triglycerides.[3][8] Lisdexamfetamine, like all pharmaceutical amphetamines, has direct appetite suppressant effects that may be therapeutically useful in both BED and its comorbidities.[4][8] Based on reviews of neuroimaging studies involving BED-diagnosed participants, therapeautic neuroplasticity in dopaminergic and noradrenergic pathways from long-term use of lisdexamfetamine may be implicated in lasting improvements in the regulation of eating behaviors that are observed even after the drug is discontinued.[4][5][8]
Narcolepsy
[edit]Narcolepsy is a chronic sleep-wake disorder that is associated with excessive daytime sleepiness, cataplexy, and sleep paralysis.[11] Patients with narcolepsy are diagnosed as either type 1 or type 2, with only the former presenting cataplexy symptoms.[12] Type 1 narcolepsy results from the loss of approximately 70,000 orexin-releasing neurons in the lateral hypothalamus, leading to significantly reduced cerebrospinal orexin levels;[13][14] this reduction is a diagnostic biomarker for type 1 narcolepsy.[12] Lateral hypothalamic orexin neurons innervate every component of the ascending reticular activating system (ARAS), which includes noradrenergic, dopaminergic, histaminergic, and serotonergic nuclei that promote wakefulness.[14][15]
Amphetamine’s therapeutic mode of action in narcolepsy primarily involves increasing monoamine neurotransmitter activity in the ARAS.[13][16][17] This includes noradrenergic neurons in the locus coeruleus, dopaminergic neurons in the ventral tegmental area, histaminergic neurons in the tuberomammillary nucleus, and serotonergic neurons in the dorsal raphe nucleus.[15][17] Dextroamphetamine, the more dopaminergic enantiomer of amphetamine, is particularly effective at promoting wakefulness because dopamine release has the greatest influence on cortical activation and cognitive arousal, relative to other monoamines.[13] In contrast, levoamphetamine may have a greater effect on cataplexy, a symptom more sensitive to the effects of norepinephrine and serotonin.[13] Noradrenergic and serotonergic nuclei in the ARAS are involved in the regulation of the REM sleep cycle and function as "REM-off" cells, with amphetamine's effect on norepinephrine and serotonin contributing to the suppression of REM sleep and a possible reduction of cataplexy at high doses.[13][12][15]
The American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline conditionally recommends dextroamphetamine for the treatment of both type 1 and type 2 narcolepsy.[18] Treatment with pharmaceutical amphetamines is generally less preferred relative to other stimulants (e.g., modafinil) and is considered a third-line treatment option.[19][20][21] Medical reviews indicate that amphetamine is safe and effective for the treatment of narcolepsy.[13][19][18] Amphetamine appears to be most effective at improving symptoms associated with hypersomnolence, with three reviews finding clinically significant reductions in daytime sleepiness in patients with narcolepsy.[13][19][18] Additionally, these reviews suggest that amphetamine may dose-dependently improve cataplexy symptoms.[13][19][18] However, the quality of evidence for these findings is low and is consequently reflected in the AASM's conditional recommendation for dextroamphetamine as a treatment option for narcolepsy.[18]
Kinase-mediated phosphorylation of DAT
[edit]Pharmacodynamics of amphetamine in a dopamine neuron
|
Amphetamine exerts its behavioral effects by altering the use of monoamines as neuronal signals in the brain, primarily in catecholamine neurons in the reward and executive function pathways of the brain.[22][29] The concentrations of the main neurotransmitters involved in reward circuitry and executive functioning, dopamine and norepinephrine, increase dramatically in a dose-dependent manner by amphetamine because of its effects on monoamine transporters.[22][29][23] The reinforcing and motivational salience-promoting effects of amphetamine are due mostly to enhanced dopaminergic activity in the mesolimbic pathway.[30] The euphoric and locomotor-stimulating effects of amphetamine are dependent upon the magnitude and speed by which it increases synaptic dopamine and norepinephrine concentrations in the striatum.[31]
Amphetamine has been identified as a potent full agonist of trace amine-associated receptor 1 (TAAR1), a Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) discovered in 2001, which is important for regulation of brain monoamines.[22][32] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits monoamine transporter function.[22][33] Based on in vitro evidence from animal models, cell lines, and human brain tissue, amphetamine appears to mediate monoamine efflux and transporter internalization in part through TAAR1 activation, which triggers the activation of protein kinases PKA, PKC, and RhoA. Amphetamine also interacts with an unidentified biomolecular target that initiates a signaling cascade involving CAMKIIα activation. Upon activation, these kinases phosphorylate their respective transporters, inducing conformational changes that alter transporter function and affect monoaminergic and glutamatergic neurotransmission at synapses. Monoamine autoreceptors (e.g., D2 short, presynaptic α2, and presynaptic 5-HT1A) have the opposite effect of TAAR1, and together these receptors provide a regulatory system for monoamines.[22][34] Notably, amphetamine and trace amines possess high binding affinities for TAAR1, but not for monoamine autoreceptors.[22][34] Preclinical imaging studies indicate that monoamine reuptake inhibition by amphetamine and trace amines is site specific and depends upon the presence of TAAR1 co-localization in the associated monoamine neurons.[22]
In addition to the neuronal monoamine transporters, amphetamine also inhibits both vesicular monoamine transporters, VMAT1 and VMAT2, as well as SLC1A1, SLC22A3, and SLC22A5.[sources 2] SLC1A1 is excitatory amino acid transporter 3 (EAAT3), a glutamate transporter located in neurons, SLC22A3 is an extraneuronal monoamine transporter that is present in astrocytes, and SLC22A5 is a high-affinity carnitine transporter.[sources 2] Amphetamine is known to strongly induce cocaine- and amphetamine-regulated transcript (CART) gene expression,[40][41] a neuropeptide involved in feeding behavior, stress, and reward, which induces observable increases in neuronal development and survival in vitro.[40][42][43] The CART receptor has yet to be identified, but there is significant evidence that CART binds to a unique Gi/Go-coupled GPCR.[43][44] Amphetamine also inhibits monoamine oxidases at very high doses, resulting in less monoamine and trace amine metabolism and consequently higher concentrations of synaptic monoamines.[45][46] In humans, the only post-synaptic receptor at which amphetamine is known to bind is the 5-HT1A receptor, where it acts as an agonist with low micromolar affinity.[47][48]
The full profile of amphetamine's short-term drug effects in humans is mostly derived through increased cellular communication or neurotransmission of dopamine,[22] serotonin,[22] norepinephrine,[22] epinephrine,[23] histamine,[23] CART peptides,[40][41] endogenous opioids,[49][50][51] adrenocorticotropic hormone,[52][53] corticosteroids,[52][53] and glutamate,[27][36] which it affects through interactions with CART, 5-HT1A, EAAT3, TAAR1, VMAT1, VMAT2, and possibly other biological targets.[sources 3] Amphetamine also activates seven human carbonic anhydrase enzymes, several of which are expressed in the human brain.[54]
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[55] Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[56][55]
Dopamine
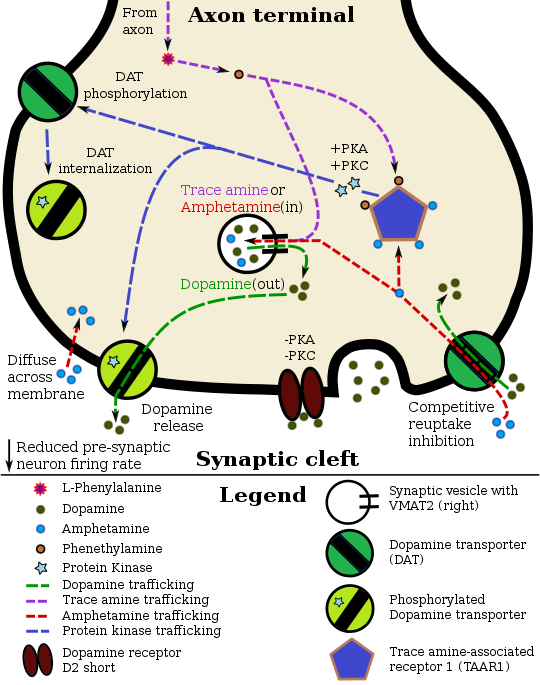
[edit]In certain brain regions, amphetamine increases the concentration of dopamine in the synaptic cleft.[22] Amphetamine can enter the presynaptic neuron either through DAT or by diffusing across the neuronal membrane directly.[22] As a consequence of DAT uptake, amphetamine produces competitive reuptake inhibition at the transporter.[22] Upon entering the presynaptic neuron, amphetamine activates TAAR1 which, through protein kinase A (PKA) and protein kinase C (PKC) signaling, causes DAT phosphorylation.[22] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces the reversal of dopamine transport through DAT (i.e., dopamine efflux).[note 1][22][57] Amphetamine is also known to increase intracellular calcium, an effect which is associated with DAT phosphorylation through an unidentified Ca2+/calmodulin-dependent protein kinase (CAMK)-dependent pathway, in turn producing dopamine efflux.[32][27][28] Through direct activation of G protein-coupled inwardly-rectifying potassium channels, TAAR1 reduces the firing rate of dopamine neurons, preventing a hyper-dopaminergic state.[25][26][58]
| Biological target of amphetamine |
Secondary effector protein kinase |
Phosphorylated transporter |
Effect on transporter function | Effect on neurotransmission | Sources |
|---|---|---|---|---|---|
| Unidentified | CAMKIIα | DAT | Reverse transport of dopamine | Dopamine efflux into synaptic cleft | [59][60][61] |
| TAAR1 | ROCK† | DAT | Transporter internalization | Dopamine reuptake inhibition | [62][63][64] |
| TAAR1 | ROCK† | EAAT3 | Transporter internalization | Glutamate reuptake inhibition | [62][63][64] |
| TAAR1 | PKA | DAT | Transporter internalization | Dopamine reuptake inhibition | [22][34] |
| TAAR1 | PKC | DAT | Reverse transport of dopamine Transporter internalization |
Dopamine efflux into synaptic cleft Dopamine reuptake inhibition |
[60][22][34] |
| †Note: ROCK-mediated transporter internalization is transient due to the inactivation of RhoA (which activates ROCK) by PKA. | [65][63][64] | ||||
Amphetamine is also a substrate for the presynaptic vesicular monoamine transporter, VMAT2.[23][24] Following amphetamine uptake at VMAT2, amphetamine induces the collapse of the vesicular pH gradient, which results in the release of dopamine molecules from synaptic vesicles into the cytosol via dopamine efflux through VMAT2.[23][24] Subsequently, the cytosolic dopamine molecules are released from the presynaptic neuron into the synaptic cleft via reverse transport at DAT.[22][23][24]
Reference notes
[edit]References
[edit]- ^ a b Giel KE, Bulik CM, Fernandez-Aranda F, Hay P, Keski-Rahkonen A, Schag K, Schmidt U, Zipfel S (March 2022). "Binge eating disorder". Nature Reviews. Disease Primers. 8 (1): 16. doi:10.1038/s41572-022-00344-y. PMC 9793802. PMID 35301358.
- ^ a b c d Heal DJ, Smith SL (June 2022). "Prospects for new drugs to treat binge-eating disorder: Insights from psychopathology and neuropharmacology". Journal of Psychopharmacology. 36 (6): 680–703. doi:10.1177/02698811211032475. PMC 9150143. PMID 34318734.
BED subjects have substantial decrements in their ventral striatal reward pathways and diminished ability to recruit fronto-cortical impulse-control circuits to implement dietary restraint. ...
There is not only substantial overlap between the psychopathology of BED and ADHD but also a clear association between these two disorders. Lisdexamfetamine's ability to reduce impulsivity and increase cognitive control in ADHD supports the hypothesis that efficacy in BED is dependent on treating its core obsessive, compulsive and impulsive behaviours. - ^ a b c d e McElroy SL (2017). "Pharmacologic Treatments for Binge-Eating Disorder". The Journal of Clinical Psychiatry. 78 Suppl 1: 14–19. doi:10.4088/JCP.sh16003su1c.03. PMID 28125174.
Genetic polymorphisms associated with abnormal dopaminergic signaling have been found in individuals who exhibit binge-eating behavior, and the binge-eating episodes,which often involve the consumption of highly palatable food, further stimulate the dopaminergic system. This ongoing stimulation may contribute to progressive impairments in dopamine signaling. Lisdexamfetamine is hypothesized to reduce binge-eating behavior by normalizing dopaminergic activity. ...
After 12 weeks, both studies found significant reductions in the number of binge-eating days per week in the active treatment group compared with placebo (P < .001 for both studies; Figure 1). Lisdexamfetamine was also found to be superior to placebo on a number of secondary outcome measures including global improvement, binge-eating cessation for 4 weeks, and reduction of obsessive-compulsive binge-eating symptoms, body weight, and triglycerides. - ^ a b c d Rodan SC, Bryant E, Le A, Maloney D, Touyz S, McGregor IS, Maguire S (July 2023). "Pharmacotherapy, alternative and adjunctive therapies for eating disorders: findings from a rapid review". Journal of Eating Disorders. 11 (1): 112. doi:10.1186/s40337-023-00833-9. PMC 10327007. PMID 37415200.
LDX is commonly used in the treatment of ADHD, and is the only treatment for BED that is currently approved by the Food and Drug Administration (FDA) and the Therapeutic Goods Administration (TGA). LDX, like all amphetamine stimulants, has direct appetite suppressant effects that may be therapeutically useful in BED, although long-term neuroadaptations in dopaminergic and noradrenergic systems caused by LDX may also be relevant, leading to improved regulation of eating behaviours, attentional processes and goal-directed behaviours. ...
Evidently, there is a substantial volume of trials with high-quality evidence supporting the efficacy of LDX in reducing binge eating frequency in treatment of adults with moderate to severe BED at 50–70 mg/day. - ^ a b c d e Boswell RG, Potenza MN, Grilo CM (January 2021). "The Neurobiology of Binge-eating Disorder Compared with Obesity: Implications for Differential Therapeutics". Clinical Therapeutics. 43 (1): 50–69. doi:10.1016/j.clinthera.2020.10.014. PMC 7902428. PMID 33257092.
Stimulant medications may be especially effective for individuals with BED because of dual effects on reward and executive function systems. Indeed, the only FDA-approved pharmacotherapy for BED is LDX, a d-amphetamine prodrug. ...
In humans, RCTs found that LDX reduced binge eating and impulsivity/compulsivity symptoms. Notably, there is a strong correlation between compulsivity symptoms and severity/frequency of binge eating episodes observed in LDX trials. Further, in individuals with BED, changes in prefrontal brain systems associated with LDX treatment were related to treatment outcome. - ^ a b c d Berry MD, Gainetdinov RR, Hoener MC, Shahid M (December 2017). "Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges". Pharmacology & Therapeutics. 180: 161–180. doi:10.1016/j.pharmthera.2017.07.002. PMID 28723415.
- ^ Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 14: Higher Cognitive Function and Behavioral Control". Molecular neuropharmacology: a foundation for clinical neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 9780071827706.
Because behavioral responses in humans are not rigidly dictated by sensory inputs and drives, behavioral responses can instead be guided in accordance with short- or long-term goals, prior experience, and the environmental context. The response to a delicious-looking dessert is different depending on whether a person is alone staring into his or her refrigerator, is at a formal dinner party attended by his or her punctilious boss, or has just formulated the goal of losing 10 lb. ...
Adaptive responses depend on the ability to inhibit automatic or prepotent responses (eg, to ravenously eat the dessert or run from the snake) given certain social or environmental contexts or chosen goals and, in those circumstances, to select more appropriate responses. In conditions in which prepotent responses tend to dominate behavior, such as in drug addiction, where drug cues can elicit drug seeking (Chapter 16), or inattention deficit hyperactivity disorder (ADHD; described below), significant negative consequences can result. - ^ a b c d e f g Schneider E, Higgs S, Dourish CT (December 2021). "Lisdexamfetamine and binge-eating disorder: A systematic review and meta-analysis of the preclinical and clinical data with a focus on mechanism of drug action in treating the disorder" (PDF). European Neuropsychopharmacology. 53: 49–78. doi:10.1016/j.euroneuro.2021.08.001. PMID 34461386.
Our meta-analysis of the four RCT data sets (Guerdjikova et al., 2016; McElroy et al., 2015b; McElroy et al., 2016a) showed an overall significant effect of LDX on binge-eating symptom change. ...
BED has been described as an impulse control disorder since one of the key symptoms of the disorder is a lack of control over eating (American Psychiatric Association, 2013) and it is possible that LDX may be effective in treating BED at least in part by reducing impulsivity, compulsivity, and the repetitive nature of binge eating. There is extensive evidence that loss of impulse control in BED is a causal factor in provoking bingeing symptoms (Colles et al., 2008; Galanti et al., 2007; Giel et al., 2017; McElroy et al., 2016a; Nasser et al., 2004; Schag et al., 2013). More specifically, BED is associated with motor impulsivity and non-planning impulsivity which could initiate and maintain binge eating (Nasser et al., 2004). Neuroimaging studies using the Stroop task to measure impulse control have shown that BED patients have decreased BOLD fMRI activity in brain areas involved in self-regulation and impulse control including VMPFC, inferior frontal gyrus (IFG), and insula during performance of the task compared to lean and obese controls (Balodis et al., 2013b). ...
It is conceivable that in BED patients a low 30 mg dose of LDX could reduce food intake by suppressing appetite or enhancing satiety and higher (50 and 70 mg) doses of the drug may have a dual suppressant effect on food intake and binge-eating frequency. - ^ Branis NM, Wittlin SD (2015). "Amphetamine-Like Analogues in Diabetes: Speeding towards Ketogenesis". Case Reports in Endocrinology. 2015: 917869. doi:10.1155/2015/917869. PMC 4417573. PMID 25960894.
Peripheral norepinephrine concentration rises as well. As demonstrated after Dextroamphetamine administration, plasma norepinephrine can rise up to 400 pg/mL, a level comparable to that achieved during mild physical activity. Cumulative effect on norepinephrine concentration is likely when amphetamine-type medications are given in the setting of acute illness or combined with activities leading to catecholamine release, such as exercise. ... The primary effect of norepinephrine on ketogenesis is mediated through increased substrate availability. As shown by Krentz et al., at high physiological concentrations, norepinephrine induces accelerated lipolysis and increases NEFA formation significantly. Secondly, norepinephrine stimulates ketogenesis directly at the hepatocyte level. As reported by Keller et al., norepinephrine infusion increased ketone bodies concentration to a greater degree when compared to NEFA concentration (155 ± 30 versus 57 ± 16%), suggesting direct hepatic ketogenic effect.
- ^ Muratore AF, Attia E (July 2022). "Psychopharmacologic Management of Eating Disorders". Current Psychiatry Reports. 24 (7): 345–351. doi:10.1007/s11920-022-01340-5. PMC 9233107. PMID 35576089.
An 11-week, double-blind RCT examined the effects of three doses of lisdexamfetamine (30 mg/day, 50 mg/day, 70 mg/day) and placebo on binge eating frequency. Results indicated that 50 mg and 70 mg doses were superior to placebo in reducing binge eating. Two follow-up 12-week RCTs confirmed the superiority of 50 and 70 mg doses to placebo in improving binge eating and secondary outcome measures, including obsessive–compulsive symptoms, body weight, and global improvement. ... Subsequent studies of lisdexamfetamine provided further support for the medication's safety and efficacy and provided additional evidence that continued use may be better than placebo in preventing relapse. While it is considered safe and effective, lisdexamfetamine's side effect profile and risk for misuse may make it inappropriate for certain patients.
- ^ Mahlios J, De la Herrán-Arita AK, Mignot E (October 2013). "The autoimmune basis of narcolepsy". Current Opinion in Neurobiology. 23 (5): 767–773. doi:10.1016/j.conb.2013.04.013. PMC 3848424. PMID 23725858.
- ^ a b c Barateau L, Pizza F, Plazzi G, Dauvilliers Y (August 2022). "Narcolepsy". Journal of Sleep Research. 31 (4): e13631. doi:10.1111/jsr.13631. PMID 35624073.
Narcolepsy type 1 was called "narcolepsy with cataplexy" before 2014 (AASM, 2005), but was renamed NT1 in the third and last international classification of sleep disorders (AASM, 2014). ... A low level of Hcrt-1 in the CSF is very sensitive and specific for the diagnosis of NT1. ...
All patients with low CSF Hcrt-1 levels are considered as NT1 patients, even if they report no cataplexy (in about 10–20% of cases), and all patients with normal CSF Hcrt-1 levels (or without cataplexy when the lumbar puncture is not performed) as NT2 patients (Baumann et al., 2014). ...
In patients with NT1, the absence of Hcrt leads to the inhibition of regions that suppress REM sleep, thus allowing the activation of descending pathways inhibiting motoneurons, leading to cataplexy. - ^ a b c d e f g h Mignot EJ (October 2012). "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics. 9 (4): 739–752. doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
At the pathophysiological level, it is now clear that most narcolepsy cases with cataplexy, and a minority of cases (5–30 %) without cataplexy or with atypical cataplexy-like symptoms, are caused by a lack of hypocretin (orexin) of likely an autoimmune origin. In these cases, once the disease is established, the majority of the 70,000 hypocretin-producing cells have been destroyed, and the disorder is irreversible. ...
Amphetamines are exceptionally wake-promoting, and at high doses also reduce cataplexy in narcoleptic patients, an effect best explained by its action on adrenergic and serotoninergic synapses. ...
The D-isomer is more specific for DA transmission and is a better stimulant compound. Some effects on cataplexy (especially for the L-isomer), secondary to adrenergic effects, occur at higher doses. ...
Numerous studies have shown that increased dopamine release is the main property explaining wake-promotion, although norepinephrine effects also contribute. - ^ a b Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. pp. 456–457. ISBN 9780071827706.
More recently, the lateral hypothalamus was also found to play a central role in arousal. Neurons in this region contain cell bodies that produce the orexin (also called hypocretin) peptides (Chapter 6). These neurons project widely throughout the brain and are involved in sleep, arousal, feeding, reward,aspects of emotion, and learning. In fact, orexin is thought to promote feeding primarily by promoting arousal. Mutations in orexin receptors are responsible for narcolepsy in a canine model, knockout of the orexin gene produces narcolepsy in mice, and humans with narcolepsy have low or absent levels of orexin peptides in cerebrospinal fluid (Chapter 13). Lateral hypothalamus neurons have reciprocal connections with neurons that produce monoamine neurotransmitters (Chapter 6).
- ^ a b c Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 13: Sleep and Arousal". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). McGraw-Hill Medical. p. 521. ISBN 9780071827706.
The ARAS consists of several different circuits including the four main monoaminergic pathways discussed in Chapter 6. The norepinephrine pathway originates from the LC and related brainstem nuclei; the serotonergic neurons originate from the RN within the brainstem as well; the dopaminergic neurons originate in the ventral tegmental area (VTA); and the histaminergic pathway originates from neurons in the tuberomammillary nucleus (TMN) of the posterior hypothalamus. As discussed in Chapter 6, these neurons project widely throughout the brain from restricted collections of cell bodies. Norepinephrine, serotonin,dopamine, and histamine have complex modulatory functions and, in general, promote wakefulness. The PT in the brainstem is also an important component of the ARAS. Activity of PT cholinergic neurons (REM-on cells) promotes REM sleep, as noted earlier. During waking, REM-on cells are inhibited by a subset of ARAS norepinephrine and serotonin neurons called REM-off cells.
- ^ Shneerson JM (2009). Sleep medicine a guide to sleep and its disorders (2nd ed.). John Wiley & Sons. p. 81. ISBN 9781405178518.
All the amphetamines enhance activity at dopamine, noradrenaline and 5HT synapses. They cause presynaptic release of preformed transmitters, and also inhibit the re-uptake of dopamine and noradrenaline. These actions are most prominent in the brainstem ascending reticular activating system and the cerebral cortex.
- ^ a b Schwartz JR, Roth T (2008). "Neurophysiology of sleep and wakefulness: basic science and clinical implications". Current Neuropharmacology. 6 (4): 367–378. doi:10.2174/157015908787386050. PMC 2701283. PMID 19587857.
Alertness and associated forebrain and cortical arousal are mediated by several ascending pathways with distinct neuronal components that project from the upper brain stem near the junction of the pons and the midbrain. ...
Key cell populations of the ascending arousal pathway include cholinergic, noradrenergic, serotoninergic, dopaminergic, and histaminergic neurons located in the pedunculopontine and laterodorsal tegmental nucleus (PPT/LDT), locus coeruleus, dorsal and median raphe nucleus, and tuberomammillary nucleus (TMN), respectively. ...
The mechanism of action of sympathomimetic alerting drugs (eg, dextro- and methamphetamine, methylphenidate) is direct or indirect stimulation of dopaminergic and noradrenergic nuclei, which in turn heightens the efficacy of the ventral periaqueductal grey area and locus coeruleus, both components of the secondary branch of the ascending arousal system. ...
Sympathomimetic drugs have long been used to treat narcolepsy - ^ a b c d e Maski K, Trotti LM, Kotagal S, Robert Auger R, Rowley JA, Hashmi SD, Watson NF (September 2021). "Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline". Journal of Clinical Sleep Medicine. 17 (9): 1881–1893. doi:10.5664/jcsm.9328. PMC 8636351. PMID 34743789.
The TF identified 1 double-blind RCT, 1 single-blind RCT, and 1 retrospective observational long-term self-reported case series assessing the efficacy of dextroamphetamine in patients with narcolepsy type 1 and narcolepsy type 2. These studies demonstrated clinically significant improvements in excessive daytime sleepiness and cataplexy.
- ^ a b c d Barateau L, Lopez R, Dauvilliers Y (October 2016). "Management of Narcolepsy". Current Treatment Options in Neurology. 18 (10): 43. doi:10.1007/s11940-016-0429-y. PMID 27549768.
The usefulness of amphetamines is limited by a potential risk of abuse, and their cardiovascular adverse effects (Table 1). That is why, even though they are cheaper than other drugs, and efficient, they remain third-line therapy in narcolepsy. Three class II studies showed an improvement of EDS in that disease. ...
Despite the potential for drug abuse or tolerance using stimulants, patients with narcolepsy rarely exhibit addiction to their medication. ...
Some stimulants, such as mazindol, amphetamines, and pitolisant, may also have some anticataplectic effects. - ^ Dauvilliers Y, Barateau L (August 2017). "Narcolepsy and Other Central Hypersomnias". Continuum. 23 (4, Sleep Neurology): 989–1004. doi:10.1212/CON.0000000000000492. PMID 28777172.
Recent clinical trials and practice guidelines have confirmed that stimulants such as modafinil, armodafinil, or sodium oxybate (as first line); methylphenidate and pitolisant (as second line [pitolisant is currently only available in Europe]); and amphetamines (as third line) are appropriate medications for excessive daytime sleepiness.
- ^ Thorpy MJ, Bogan RK (April 2020). "Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications". Sleep Medicine. 68: 97–109. doi:10.1016/j.sleep.2019.09.001. PMID 32032921.
The first agents used to treat EDS (ie, amphetamines, methylphenidate) are now considered second- or third-line options because newer medications have been developed with improved tolerability and lower abuse potential (eg, modafinil/armodafinil, solriamfetol, pitolisant)
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468. Cite error: The named reference "Miller" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). ... AMPH release of DA from synapses requires both an action at VMAT2 to release DA to the cytoplasm and a concerted release of DA from the cytoplasm via "reverse transport" through DAT.
Cite error: The named reference "E Weihe" was defined multiple times with different content (see the help page). - ^ a b c d Sulzer D, Cragg SJ, Rice ME (August 2016). "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia. 6 (3): 123–148. doi:10.1016/j.baga.2016.02.001. PMC 4850498. PMID 27141430.
Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
Cite error: The named reference "Amphetamine VMAT2 pH gradient" was defined multiple times with different content (see the help page). - ^ a b Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (July 2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
Cite error: The named reference "GIRK" was defined multiple times with different content (see the help page). - ^ a b "TAAR1". GenAtlas. University of Paris. 28 January 2012. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)
Cite error: The named reference "Genatlas TAAR1" was defined multiple times with different content (see the help page). - ^ a b c d Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
Cite error: The named reference "EAAT3" was defined multiple times with different content (see the help page). - ^ a b Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
Cite error: The named reference "DAT regulation review" was defined multiple times with different content (see the help page). - ^ a b Cite error: The named reference
cognition enhancerswas invoked but never defined (see the help page). - ^ Cite error: The named reference
Malenka_2009was invoked but never defined (see the help page). - ^ Cite error: The named reference
Amph Useswas invoked but never defined (see the help page). - ^ a b Maguire JJ, Davenport AP (2 December 2014). "TA1 receptor". IUPHAR database. International Union of Basic and Clinical Pharmacology. Archived from the original on 29 June 2015. Retrieved 8 December 2014.
- ^ Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (July 2001). "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proceedings of the National Academy of Sciences. 98 (16): 8966–8971. Bibcode:2001PNAS...98.8966B. doi:10.1073/pnas.151105198. PMC 55357. PMID 11459929.
- ^ a b c d Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug Alcohol Depend. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMC 4724540. PMID 26644139.
TAAR1 activation modulates monoamine transporters via PKA signaling among other pathways, altering dopamine uptake and release.
- ^ a b "SLC18 family of vesicular amine transporters". IUPHAR database. International Union of Basic and Clinical Pharmacology. Retrieved 13 November 2015.
- ^ a b c "SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 [ Homo sapiens (human) ]". NCBI Gene. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 11 November 2014.
Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. ... internalization of EAAT3 triggered by amphetamine increases glutamatergic signaling and thus contributes to the effects of amphetamine on neurotransmission.
- ^ Zhu HJ, Appel DI, Gründemann D, Markowitz JS (July 2010). "Interaction of organic cation transporter 3 (SLC22A3) and amphetamine". Journal of Neurochemistry. 114 (1): 142–149. doi:10.1111/j.1471-4159.2010.06738.x. PMC 3775896. PMID 20402963.
- ^ Rytting E, Audus KL (January 2005). "Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells". Journal of Pharmacology and Experimental Therapeutics. 312 (1): 192–198. doi:10.1124/jpet.104.072363. PMID 15316089. S2CID 31465243.
- ^ Inazu M, Takeda H, Matsumiya T (August 2003). "[The role of glial monoamine transporters in the central nervous system]". Nihon Shinkei Seishin Yakurigaku Zasshi (in Japanese). 23 (4): 171–178. PMID 13677912.
- ^ a b c Cite error: The named reference
Drugbank-amphwas invoked but never defined (see the help page). - ^ a b c Vicentic A, Jones DC (February 2007). "The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction". Journal of Pharmacology and Experimental Therapeutics. 320 (2): 499–506. doi:10.1124/jpet.105.091512. PMID 16840648. S2CID 14212763.
The physiological importance of CART was further substantiated in numerous human studies demonstrating a role of CART in both feeding and psychostimulant addiction. ... Colocalization studies also support a role for CART in the actions of psychostimulants. ... CART and DA receptor transcripts colocalize (Beaudry et al., 2004). Second, dopaminergic nerve terminals in the NAc synapse on CART-containing neurons (Koylu et al., 1999), hence providing the proximity required for neurotransmitter signaling. These studies suggest that DA plays a role in regulating CART gene expression possibly via the activation of CREB.
- ^ Zhang M, Han L, Xu Y (June 2012). "Roles of cocaine- and amphetamine-regulated transcript in the central nervous system". Clinical and Experimental Pharmacology and Physiology. 39 (6): 586–592. doi:10.1111/j.1440-1681.2011.05642.x. PMID 22077697. S2CID 25134612.
Recently, it was demonstrated that CART, as a neurotrophic peptide, had a cerebroprotective against focal ischaemic stroke and inhibited the neurotoxicity of β-amyloid protein, which focused attention on the role of CART in the central nervous system (CNS) and neurological diseases. ... The literature indicates that there are many factors, such as regulation of the immunological system and protection against energy failure, that may be involved in the cerebroprotection afforded by CART
- ^ a b Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ (October 2008). "CART peptides: regulators of body weight, reward and other functions". Nature Reviews Neuroscience. 9 (10): 747–758. doi:10.1038/nrn2493. PMC 4418456. PMID 18802445.
Several studies on CART (cocaine- and amphetamine-regulated transcript)-peptide-induced cell signalling have demonstrated that CART peptides activate at least three signalling mechanisms. First, CART 55–102 inhibited voltage-gated L-type Ca2+ channels ...
- ^ Lin Y, Hall RA, Kuhar MJ (October 2011). "CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6–38 as a CART receptor antagonist". Neuropeptides. 45 (5): 351–358. doi:10.1016/j.npep.2011.07.006. PMC 3170513. PMID 21855138.
- ^ "Compound Summary". Amphetamine. United States National Library of Medicine – National Center for Biotechnology Information. PubChem Compound Database. 11 April 2015. Retrieved 17 April 2015.
- ^ Monoamine oxidase (Homo sapiens). Technische Universität Braunschweig. BRENDA. 1 January 2014. Retrieved 4 May 2014.
- ^ a b Cite error: The named reference
5HT1A secondarywas invoked but never defined (see the help page). - ^ Cite error: The named reference
5HT1A Primarywas invoked but never defined (see the help page). - ^ Finnema SJ, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, Sallinen J, Wong E, Farde L, Halldin C, Grimwood S (November 2015). "Application of cross-species PET imaging to assess neurotransmitter release in brain". Psychopharmacology. 232 (21–22): 4129–4157. doi:10.1007/s00213-015-3938-6. PMC 4600473. PMID 25921033.
More recently, Colasanti and colleagues reported that a pharmacologically induced elevation in endogenous opioid release reduced [11C]carfentanil binding in several regions of the human brain, including the basal ganglia, frontal cortex, and thalamus (Colasanti et al. 2012). Oral administration of d-amphetamine, 0.5 mg/kg, 3 h before [11C]carfentanil injection, reduced BPND values by 2–10%. The results were confirmed in another group of subjects (Mick et al. 2014). However, Guterstam and colleagues observed no change in [11C]carfentanil binding when d-amphetamine, 0.3 mg/kg, was administered intravenously directly before injection of [11C]carfentanil (Guterstam et al. 2013). It has been hypothesized that this discrepancy may be related to delayed increases in extracellular opioid peptide concentrations following amphetamine-evoked monoamine release (Colasanti et al. 2012; Mick et al. 2014).
- ^ Loseth GE, Ellingsen DM, Leknes S (December 2014). "State-dependent μ-opioid modulation of social motivation". Frontiers in Behavioral Neuroscience. 8: 430. doi:10.3389/fnbeh.2014.00430. PMC 4264475. PMID 25565999.
Similar MOR activation patterns were reported during positive mood induced by an amusing video clip (Koepp et al., 2009) and following amphetamine administration in humans (Colasanti et al., 2012).
- ^ Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, Waldman AD, Schruers KR, Matthews PM, Gunn RN, Nutt DJ, Rabiner EA (September 2012). "Endogenous opioid release in the human brain reward system induced by acute amphetamine administration". Biological Psychiatry. 72 (5): 371–377. doi:10.1016/j.biopsych.2012.01.027. PMID 22386378. S2CID 18555036.
- ^ a b Cite error: The named reference
Human amph effectswas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Primary: Human HPA axiswas invoked but never defined (see the help page). - ^ Cite error: The named reference
Amphetamine-induced activation of 7 hCA isoformswas invoked but never defined (see the help page). - ^ a b Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorganic & Medicinal Chemistry. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ Cite error: The named reference
Westfallwas invoked but never defined (see the help page). - ^ Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (March 2009). "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacological Reviews. 61 (1): 1–8. doi:10.1124/pr.109.001107. PMC 2830119. PMID 19325074.
- ^ Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (May 2011). "TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity". Proceedings of the National Academy of Sciences. 108 (20): 8485–8490. doi:10.1073/pnas.1103029108. PMC 3101002. PMID 21525407.
- ^ Steinkellner T, Mus L, Eisenrauch B, Constantinescu A, Leo D, Konrad L, Rickhag M, Sørensen G, Efimova EV, Kong E, Willeit M, Sotnikova TD, Kudlacek O, Gether U, Freissmuth M, Pollak DD, Gainetdinov RR, Sitte HH (October 2014). "In vivo amphetamine action is contingent on αCaMKII". Neuropsychopharmacology. 39 (11): 2681–2693. doi:10.1038/npp.2014.124. PMC 4207348. PMID 24871545.
Our findings demonstrate that amphetamine requires the presence of αCaMKII to elicit a full-fledged effect on DAT in vivo: αCaMKII does not only support acute amphetamine-induced dopamine efflux but is also important in shaping the chronic response to amphetamine.
- ^ a b Wang Q, Bubula N, Brown J, Wang Y, Kondev V, Vezina P (May 2016). "PKC phosphorylates residues in the N-terminal of the DA transporter to regulate amphetamine-induced DA efflux". Neurosci. Lett. 622: 78–82. doi:10.1016/j.neulet.2016.04.051. PMC 4870132. PMID 27113203.
The DA transporter (DAT), a phosphoprotein, controls extracellular dopamine (DA) levels in the central nervous system through transport or reverse transport (efflux). Multiple lines of evidence support the claim that PKC significantly contributes to amphetamine-induced DA efflux. Other signaling pathways, involving CaMKII and ERK, have also been shown to regulate DAT mediated efflux. ... The results of in vitro experiments using a recombinant N-terminal peptide of DAT [11,17] indicate that PKC phosphorylates the S4, S7, and S13 residues, that the S7 and S13 residues are also phosphorylated by PKA and CaMKII respectively, and that the T53 residue is phosphorylated by ERK1/2 (Fig. 1). ... Together, these findings suggest that PKC is not the only protein kinase that regulates amphetamine-induced DA efflux and, importantly, that it may function in concert with others at multiple residues in the N-terminal of the DAT to fully regulate its function. ... DA efflux is regulated by several kinases in addition to PKC, including CaMKII and ERK1/2 [5,6], and all are capable of regulating the DAT by phosphorylating residues in its N-terminal [11–15,17].
- ^ Bermingham DP, Blakely RD (October 2016). "Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters". Pharmacol. Rev. 68 (4): 888–953. doi:10.1124/pr.115.012260. PMC 5050440. PMID 27591044.
The Amara laboratory recently provided evidence that AMPH triggered DAT endocytosis is clathrin-independent and requires the small GTPase Rho (Wheeler et al., 2015)... Whereas little support for CaMKII regulation of DA uptake exists, substantial evidence supports a role for the kinase in DAT-dependent DA efflux triggered by AMPH... AMPH was shown to activate CaMKII in DAT transfected cells (Wei et al., 2007). ... At present, information is lacking as to the site(s) that support CaMKII phosphorylation of DAT in vivo ... The current model... DAT by phosphorylating one or more Ser residues in the transporter N terminus. This phosphorylation is then thought to facilitate conformational changes that place the transporter in a "DA efflux-willing" conformation.
- ^ a b Underhill SM, Hullihen PD, Chen J, Fenollar-Ferrer C, Rizzo MA, Ingram SL, Amara SG (August 2019). "Amphetamines signal through intracellular TAAR1 receptors coupled to Gα13 and GαS in discrete subcellular domains". Mol. Psychiatry. doi:10.1038/s41380-019-0469-2. PMID 31399635.
Figure 6: Amphetamine signling through intracellular TAAR1 receptors
{{cite journal}}: External link in|quote= - ^ a b c Wheeler DS, Underhill SM, Stolz DB, Murdoch GH, Thiels E, Romero G, Amara SG (December 2015). "Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine". Proc. Natl. Acad. Sci. U.S.A. 112 (51): E7138 – E7147. doi:10.1073/pnas.1511670112. PMC 4697400. PMID 26553986.
These observations support the existence of an unanticipated intracellular target that mediates the effects of AMPH on RhoA and cAMP signaling and suggest new pathways to target to disrupt AMPH action. ... Using a ROCK inhibitor, Y27632, blocked the effects of AMPH pretreatment on dopamine uptake... The activation of intracellular signaling pathways by AMPH and the Rho-mediated internalization of DAT are also observed in nonneural cell lines... Cytoplasmic cAMP appears to integrate both intracellular signals through GTPase activation and extracellular signals from GPCR-coupled pathways... Thus, modulation of the Rho activation/inactivation sequence provides a mechanism by which drugs and endogenous neurotransmitters can influence the response of dopamine neurons to AMPH.
- ^ a b c Saunders C, Galli A (December 2015). "Insights in how amphetamine ROCKs (Rho-associated containing kinase) membrane protein trafficking". Proc. Natl. Acad. Sci. U.S.A. 112 (51): 15538–15539. doi:10.1073/pnas.1520960112. PMC 4697384. PMID 26607447.
In this elegant and thorough study (7), Amara and her collaborators identify multiple novel targets for intracellular AMPH. They demonstrate that cytoplasmic AMPH stimulates a secondary pathway of cAMP production, which leads to Rho inactivation by PKA-dependent phosphorylation. ... ROCK inhibition blocks the effects of AMPH pretreatment on DA uptake, supporting previous studies suggesting a role for ROCK in AMPH's behavioral effects... These results further support the idea that direct activation of cytoplasmic signaling cascades by AMPH might contribute to the behavioral effects of acute AMPH exposure.
- ^ Bjørn-Yoshimoto WE, Underhill SM (September 2016). "The importance of the excitatory amino acid transporter 3 (EAAT3)". Neurochem. Int. 98: 4–18. doi:10.1016/j.neuint.2016.05.007. PMC 4969196. PMID 27233497.
Recently, it was reported that amphetamine decreases the surface expression of EAAT3 (Underhill et al., 2014). This was dependent on RhoA activation. Interestingly, it was also reported that the dopamine transporter follows the same RhoA dependent mechanism of amphetamine-induced endocytosis (Wheeler et al., 2015).
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).