User:Matteo lamparella/sandbox
Tail Of The Bacteriophages
[edit]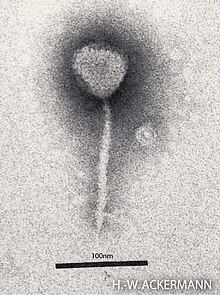
the bacteriophage entity very present in the earth,is composed of a tail that can be of various types that allows the entity to recognize the host and deliver the DNA from the capsid to the cytoplasm of the infected cell regardless of their gram positive or gram negative host, or their receptor nature or the type of polysaccharide or protein surface in fact the central tail architecture of all caudophages and phage derivates, the systems share the same structural organization and are believed to be be homologous.
here we review recent advances in the structure, function and assembly of the phage tail architecture.
in fact, we have three categories of the phages also classified according to the type of tail, such as siphovoridae (long flexible tail) then myoviridae (long contractual tail) and podovoridae (short tail). The path to mount the capsid and also the tail are indipendent. The capsids full of DNA with the tails assembling form the complete virion, which is released with cell lysis.
Because there is an interaction with the cell surface, there is a trigger for infection in the host cell. The phage tail is still a highly sought after and interesting subject of study especially in terms of perforation of the cell wall, in fact the tail has precisely the task of the delivering the genome into the bacterium cytoplasm. So the tail is a multy-protein and complex structure that mediates the expulsion of the genome, little is known about how the tail can pass through the last layer that protects the cytoplasm of the host cell, images from cryoelectric microscopy (cryo-em) show that phages use the tail to form a pore through which the genome enters the bacterial cell.
SIPHOPHAGE TAIL ANALYSIS
[edit]thus at its distal end, the tip of the tail complex is equipped with receptor-bound proteins (RBP),which are more present in one or more copies and tail fibers may also be present in siphophages. The core of the tail complex is formed by a ring of the hexameric protein of the distal tail protein (DTP), and a trimeric ring of the "HUB" protein of the baseplate (BHP), Then we have in the proximal side a long tube formed by oligomerization of the tail tube protein (TTP) around the (TMP) tape measure protein.
This tube ends with the terminator protein or in some cases the completion protein. In myophages the tail is wrapped in a sheath.
Tail Assembly Of The SIPHOPHAGES
[edit]During tail assembly, the long TMP is stabilized by the chaperones and in most phages,two proteins named by IG and GT, are synthesized and via a programmed translational frameshift -1. The effectiveness of frameshift determines the ratio of G/GT which has been shown to be a crucial for proper tail assembly. Structural analyzes suggest that G coils the TMP in a spiral mode. the polymerization of TTP around the TMP has also been demonstred which would transform the TTP into an oligomerization core. It has been explained that the TMp is folden into the tail tube in a metastable conformation, which would relax after host binding, into a lower energy conformation leading to facilitated ejection from the tail and expulsion of TMP would trigger the release of DNA from the capsid. C-terminal proteomysis could induce a transition from a chaperone bound state to a metastable TTP.
the tail tip complex located at the distal end of the tail,the tip complex is the fagus guest recogniton apparatus .although very different in size and shape, from large base plate-like sructures to very small ones, the tip comprises two structurally conserved proteins, BHP or TAL and DTP or DIT, which are also the nucleos of the phages and system of injection beat us related phages. The tip complex acts as the HUB for the RBP, or at the end of the central fiber. Over the past decade, several structures of the siphophagus tip have become available, often through a combination of EM and X-ray crystallography. for example in the "p2" phages the central role of the DTP/BHP oligomerization and assembly together with the TMP/TAC complex,which appears to be the initiator complex. The assembly of the initiator complex is followed by the polymerization of the TTP to form a tube and the attachment of peripheral proteins to the base plate . The RBPs were made developed to adapt to very different cell surfaces from the hosts.
TAIL OF THE SIPHOVIRIDAE CATEGORY
[edit]The long tail of this type of phages acts as a molecular machine that expels viral DNA from the capsid into the host cell's cytoplasm. The ejection system is made up of a central tube, base plate and a terminator assembly that fixes the tail of the phage capsid. After having bonded to the surface the baseplate with coformation and triggers expulsion of the genome in the cytoplasm of the host cell, it is said that the source of the forces that causes the expulsion of the viral genome to be due to an osmotic pressure imbalance between the virus and the interior of the host cytoplasm.
way to prenetrate in gram positive and gram negative bacteria
[edit]GRAM POSITIVE: among the phages of the siphages we have the attachment to a receptor of the outer membrane of the cell that is infected, the tip of the tail penetrates the OM of the host and the exolysin attached to the virion (if present) hydrolyzes the peptydoglycan state. the tail reaches the host's plasma membrane, through which a pore opens for the translocation of viral DNA.
GRAM NEGATIVE: Attachment to a host cell wall receptor occurs and hydrolysis is associeted with the tip of the peptydoglyca layer. The tail reaches the plasma membrane of the infected cell and a loss of the tip is possible with subsequent opening of the cap and use of a hydrophilic pore for the translocation of viable DNA into the host cytoplasm.
Note
[edit]Bibliography
[edit]- 1.** Veesler D, Cambillau C: A Common Evolutionary Origin for Tailed-Bacteriophage|
Functional Modules and Bacterial Machineries. Microbiol Mol Biol Rev MMBR 2011, 75:423–433.
- 2.** Davidson AR, Cardarelli L, Pell LG, Radford DR, Maxwell KL: Long noncontractile|
tail machines of bacteriophages. Adv Exp Med Biol 2012, 726:115–142. Extensive review that summarises many decades worth of research that cover a broad range of topics concerning the non-contractile tails of bacteriophages
- 3. Auzat I, Petitpas I, Lurz R, Weise F, Tavares P: A touch of glue to complete|
bacteriophage assembly: the tail-to-head joining protein (THJP) family. Mol Microbiol 2014, 91:1164–1178.
- Zivanovic Y, Confalonieri F, Ponchon L, Lurz R, Chami M, Flayhan A, Renouard M|,
Huet A, Decottignies P, Davidson AR, et al.: Insights into bacteriophage T5 structure from analysis of its morphogenesis genes and protein components. J Virol 2014, 88:1162–1174
- Iwasaki T, Yamashita E, Nakagawa A, Enomoto A, Tomihara M, Takeda S: Threedimensional|
structures of bacteriophage neck subunits are shared in Podoviridae, Siphoviridae and Myoviridae. Genes Cells Devoted Mol Cell Mech 2018, 23:528–536
- Chaban Y, Lurz R, Brasilès S, Cornilleau C, Karreman M, Zinn-Justin S, Tavares P|,
Orlova EV: Structural rearrangements in the phage head-to-tail interface during assembly and infection. Proc Natl Acad Sci U S A 2015, 112:7009–7014
- Fokine A, Zhang Z, Kanamaru S, Bowman VD, Aksyuk AA, Arisaka F, Rao VB|,
Rossmann MG: The molecular architecture of the bacteriophage T4 neck. J Mol Biol 2013, 425:1731–1744
- Lopes A, Tavares P, Petit M-A, Guérois R, Zinn-Justin S: Automated classification of|
tailed bacteriophages according to their neck organization. BMC Genomics 2014, 15:1027
- Tavares P: The Bacteriophage Head-to-Tail Interface. Subcell Biochem 2018, 88:305–328
- Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR: The phage lambda|
major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc Natl Acad Sci U S A 2009, 106:4160–4165.
- Zinke M, Fricke P, Samson C, Hwang S, Wall JS, Lange S, Zinn-Justin S, Lange A:|
Bacteriophage Tail-Tube Assembly Studied by Proton-Detected 4D Solid-State NMR. Angew Chem Int Ed Engl 2017, 56:9497–9501.
- Arnaud C-A, Effantin G, Vivès C, Engilberge S, Bacia M, Boulanger P, Girard E,|
Schoehn G, Breyton C: Bacteriophage T5 tail tube structure suggests a trigger mechanism for Siphoviridae DNA ejection. Nat Commun 2017, 8:1953. First pseudo-atomic structure of the tail tube of a siphophage, before and after interaction with the recpetor.
- Campbell PL, Duda RL, Nassur J, Conway JF, Huet A: Mobile Loops and Electrostatic|
Interactions Maintain the Flexible Tail Tube of Bacteriophage Lambda. J Mol Biol 2020, 432:384–395.
- Taylor NMI, van Raaij MJ, Leiman PG: Contractile injection systems of|
bacteriophages and related systems. Mol Microbiol 2018, 108:6–15. Reviews the similarities and differences between myophages and bacterial phage-derived contractile injection systems
- Langlois C, Ramboarina S, Cukkemane A, Auzat I, Chagot B, Gilquin B, Ignatiou A,|
Petitpas I, Kasotakis E, Paternostre M, et al.: Bacteriophage SPP1 tail tube protein selfassembles into β-structure-rich tubes. J Biol Chem 2015, 290:3836–3849.
- Špakova A, Šimoliūnas E, Batiuškaitė R, Pajeda S, Meškys R, Petraitytė-Burneikienė R:|
Self-Assembly of Tail Tube Protein of Bacteriophage vB_EcoS_NBD2 into Extremely Long Polytubes in E. coli and S. cerevisiae. Viruses 2019, 11.
- Plisson C, White HE, Auzat I, Zafarani A, São-José C, Lhuillier S, Tavares P, Orlova|
EV: Structure of bacteriophage SPP1 tail reveals trigger for DNA ejection. EMBO J 2007, 26:3720–3728.
- Fraser JS, Maxwell KL, Davidson AR: Immunoglobulin-like domains on|
bacteriophage: weapons of modest damage? Curr Opin Microbiol 2007, 10:382–387
- Auzat I, Dröge A, Weise F, Lurz R, Tavares P: Origin and function of the two major|
tail proteins of bacteriophage SPP1. Mol Microbiol 2008, 70:557–569
- Fraga H, Arnaud C-A, Gauto DF, Audin MJC, Kurauskas V, Macek P, Krichel C, Guan|
J-Y, Boisbouvier J, Sprangers R, et al.: Solid-state NMR H-N-(C)-H and H-N-C-C 3D/4D correlation experiments for resonance assignment of large proteins. Chemphyschem Eur J Chem Phys Phys Chem 2017, doi:10.1002/cphc.201700572
- Kizziah JL, Manning KA, Dearborn AD, Dokland T: Structure of the host cell|
recognition and penetration machinery of a Staphylococcus aureus bacteriophage. PLoS Pathog 2020, 16:e1008314.
- Katsura I: Determination of bacteriophage lambda tail length by a protein ruler.|
Nature 1987, 327:73–75
- Mahony J, Alqarni M, Stockdale S, Spinelli S, Feyereisen M, Cambillau C, Sinderen D|
van: Functional and structural dissection of the tape measure protein of lactococcal phage TP901-1. Sci Rep 2016, 6:36667
- Boulanger P, Jacquot P, Plançon L, Chami M, Engel A, Parquet C, Herbeuval C, Letellier|
L: Phage T5 straight tail fiber is a multifunctional protein acting as a tape measure and carrying fusogenic and muralytic activities. J Biol Chem 2008, 286:13556–13564.
- Tsui LC, Hendrix RW: Proteolytic processing of phage lambda tail protein gpH:|
timing of the cleavage. Virology 1983, 125:257–264.
- Xu J, Hendrix RW, Duda RL: A balanced ratio of proteins from gene G and|
frameshift-extended gene GT is required for phage lambda tail assembly. J Mol Biol 2013, 425:3476–3487.
- Pell LG, Cumby N, Clark TE, Tuite A, Battaile KP, Edwards AM, Chirgadze NY,|
Davidson AR, Maxwell KL: A conserved spiral structure for highly diverged phage tail assembly chaperones. J Mol Biol 2013, 425:2436–2449.
- Xu J, Hendrix RW, Duda RL: Chaperone-protein interactions that mediate assembly|
of the bacteriophage lambda tail to the correct length. J Mol Biol 2014, 426:1004–1018. Both Xu et al. (2013 and 2014) papers dissect the role of both tail chaperones in tail assembly and TTP polymerisation.
- Cumby N, Reimer K, Mengin-Lecreulx D, Davidson AR, Maxwell KL: The phage tail|
tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Mol Microbiol 2015, 96:437–447.
- Bebeacua C, Lorenzo Fajardo JC, Blangy S, Spinelli S, Bollmann S, Neve H, Cambillau|
C, Heller KJ: X-ray structure of a superinfection exclusion lipoprotein from phage TP-J34 and identification of the tape measure protein as its target. Mol Microbiol 2013, 89:152–165.
- Bebeacua C, Lai L, Vegge CS, Brøndsted L, van Heel M, Veesler D, Cambillau C:|
Visualizing a complete Siphoviridae member by single-particle electron microscopy: the structure of lactococcal phage TP901-1. J Virol 2013, 87:1061–1068.
- Legrand P, Collins B, Blangy S, Murphy J, Spinelli S, Gutierrez C, Richet N,|
Kellenberger C, Desmyter A, Mahony J, et al.: The Atomic Structure of the Phage Tuc2009 Baseplate Tripod Suggests that Host Recognition Involves Two Different Carbohydrate Binding Modules. mBio 2016, 7:e01781-01715.
- Goulet A, Lai-Kee-Him J, Veesler D, Auzat I, Robin G, Shepherd DA, Ashcroft AE,|
Richard E, Lichière J, Tavares P, et al.: The Opening of the SPP1 Bacteriophage Tail, a Prevalent Mechanism in Gram-positive-infecting Siphophages. J Biol Chem 2011, 286:25397–25405.
- Veesler D, Spinelli S, Mahony J, Lichière J, Blangy S, Bricogne G, Legrand P, Ortiz-|
Lombardia M, Campanacci V, van Sinderen D, et al.: Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc Natl Acad Sci U S A 2012, 109:8954–8958.
- Sciara G, Bebeacua C, Bron P, Tremblay D, Ortiz-Lombardia M, Lichière J, van Heel M,|
Campanacci V, Moineau S, Cambillau C: Structure of lactococcal phage p2 baseplate and its mechanism of activation. Proc Natl Acad Sci U S A 2010, 107:6852–6857. First atomic structure of an over-expressed siphophage baseplate, compared to a low-resolution envelop of the in phage baseplate. Structure comparison the baseplate with and without Ca2+ - mandatory for infection- reveal the activation mechanism of phage p2.
- Shepherd DA, Veesler D, Lichière J, Ashcroft AE, Cambillau C: Unraveling lactococcal|
phage baseplate assembly by mass spectrometry. Mol Cell Proteomics MCP 2011, 10:M111.009787.
Voci correlate
[edit]microbiology virology bacteriophage podoviridae
Altri progetti
[edit]Collegamenti esterni
[edit]{{cell|COLOR}}
- ^ a b Cite error: The named reference
https://hal.archives-ouvertes.fr/hal-02921467/documentwas invoked but never defined (see the help page). - ^ Cite error: The named reference
https://upload.wikimedia.org/wikipedia/commons/2/2d/Lambda_EM.jpgwas invoked but never defined (see the help page).

