User:Lykchiniadis/sandbox
Main page Peptide Loading Complex (PLC)
Composition
[edit]The peptide-loading complex (PLC) [1] is a short-lived, multisubunit protein complex that is located on the cell membrane in the endoplasmic reticulum (ER) and plays a crucial role in intriguing a hierarchical immune response. The PLC orchestrates in transporting peptides into the ER with complexing and editing of major histocompatibility class I (MHC-I) protein complex.
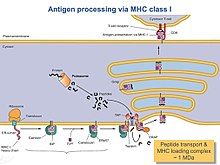
Process of immune response consists of the release of stable peptide-MHC I heterodimers to the cell surface to promote a T-cell response against malignant cells. In turn, T-cells are recognizing the activated peptides (T-cell epitopes) and the latter are functioning as immunogenic and not-immunogenic ones. The PLC assembly consists of seven different subunits including the transporter associated with antigen processing (TAP1 and TAP2, jointly referred to as TAP), the oxidoreductase ERp57, the MHC-I heterodimer, and the chaperones tapasin, calnexin, calreticulin. Dynamics of this complex system are playing a crucial role in the mechanism of function in general. Also, the heterogeneity of PLC is substantial for the understanding of the general molecular organization and mechanistic events that orchestrate the function.
Structural features
[edit]The only human PLC structure so far has been solved by single-particle electron cryo-microscopy. The structural organization consists of tapasin, calreticulin, ERp57, and MHC-I molecules that are centered around TAP bearing a pseudo-symmetric orientation. Two endoplasmic reticulum-resident editing modules composed of tapasin, calreticulin, ERp57, and MHC-I are centered around TAP in a pseudo-symmetric orientation. PLC of size 150 Å by 150 Å and has a total height of 240 Å across the ER membrane. Two tapasin molecules shape the central domain in the PLC. In the electron cryo-microscopy structure, Dr. Tampe’s group found that E225 of the N-terminal of the N-terminal immunoglobulin-like domain of one molecule interacts with R60, that is located in the short helical structural element of the seven-stranded N-terminal β barrel of the other molecule, in salt-bridge distance. The residues E225 and R60 are conserved in vertebrates.
TAP
[edit]TAP is a heterodimeric complex, consisting of TAP1 (ABCB2) and TAP2 (ABCB3) members of ABC transporter superfamily. The common feature of all ABC transporters is their organization: 1) into two transmembrane domains (TMDs) and 2) into two nucleotide-binding domains (NBDs). Both intramolecular domains are coupled to each other and when ATP binding is in process, conformational changes in the TMDs allow proteasomal degradation products to move across the membrane. TAP recognizes and transports the antigen peptides produced in cytosol straight into the ER, while tapasin recognizes the kind of peptides that have the ability to form stable complexes with MHC-I. The latter procedure is named peptide proofreading or peptide editing. Selected peptides through peptide proofreading [2] improve MHC-I stability and tapasin also contributes to the editing of immunogenic peptide epitopes. However, only lately was proved via biochemical, biophysical, and structural studies that a key function in adaptive immunity, the catalytic mechanism of peptide proofreading performed by tapasin and especially TAPBPR (TAP-binding protein-related, a tapasin homologue).
Tapasin
[edit]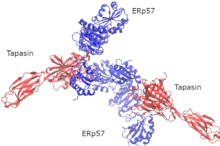
ERp57
[edit]ERp57 is an enzyme of the thiol oxidoreductase family located in the endoplasmic reticulum. It is attached to substrates in an indirect fashion through association with the molecular chaperones calnexin and calreticulin of the Peptide-loading complex. In early stages of generation of MHC-I molecules, ERp57 is associated with free MHC-I heavy chains. As a result, it’s function is determined by the formation of disulfide bonds in heavy chains, by oxidative folding of the heavy chain, and finally by the fact that ERp57 is loading the peptides onto MHC-I molecules.
Calnexin
[edit]Calreticulin
[edit]Calreticulin interacts with MHC-I and especially with the lectin-like domain of calreticulin. The P domain is facing the MHC-I peptide-binding site towards ERp57. This orientation makes it possible for tapasin to attach and secure MHC-I. This translocation of TAP facilitates the opening out into an ER cavity, edged by the standard membrane entry points such as i) tapasin and ii) MHC-I transmembrane points. This arrangement allows tapasin to facilitate peptide editing by clamping MHC-I.

The translocation pathway of TAP opens out into a large endoplasmic reticulum lumenal cavity, confined by the membrane entry points of tapasin and MHC-I. These two entry points facilitate the recruitment of MHC-I with optimal peptide loading and in a final stage the release of MHC-I in T-cells surface for recognition.
TAPBPR
[edit]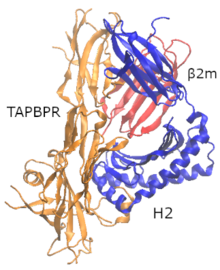
Proofreading of high-affinity peptide epitopes on MHC-I complex is substantial for T-cell differentiation. In 2017, Dr. Tampé [3] found a PLC-independent tapasin homologue protein named TAPBPR that has the ability to act as a second MHC-I specific peptide proofreader or editor but does not possess a transmembrane domain. Tapasin and TAPBPR share similar binding interfaces on MHC-I, as shown with the X-ray structure of TAPBPR with MHC-I (heavy chain (hc) and β2 microglobulin (β2m)). The use of a photo-cleavable high-affinity peptide allowed the researchers to form a stable (bound) MHC-I molecules and afterwards to form a stable TAPBPR and MHC-I complex with cleavage by UV light of the photoinduced peptide.
MHC-I
[edit]Preliminary MHC-I heavy chains are forming chaperones with the aid of calnexin-calreticulin complex in the ER. In addition to this, β2-microglobulin (β2m) is attached to the heavy chains of the heterodimers and as a whole they act as receptors for antigenic peptides. When MHC-I are empty, they are recruited by calreticulin and form the transient PLC. Tapasin is always playing a role of stabilization of MHC-I. Only after MHC-I heterodimers are in the process of proofreading or peptide editing, stable pMHC-I (peptide-MHC-I) complexes are released to the cell surface for recognition and destruction of virally or malignantly transformed T-cells. Amino acids positions P4-6 of the peptide and also large and aromatic side chains, possibly are better recognized by T-cells [4]. In general, each person owns a collection of six MHC-I molecules (three from each parent). So in emergency cases of triggering an immune response to destroy the cells, one has to find a compatible donor, a relative who owns a similar collection of MHC-I molecules, except of his own MHC-I molecules.
References
[edit]- ^ Andreas Blees, Dovile Januliene, Tommy Hofmann, Nicole Koller, Carla Schmidt, Simon Trowitzsch, Arne Moeller & Robert Tampé, Structure of the human MHC-I peptide-loading complex, Nature, 2017, 551, 525-528, doi: https://doi.org/10.1038/nature24627
- ^ Christoph Thomas and Robert Tampé, Proofreading of Peptide—MHC Complexes through Dynamic Multivalent Interactions, Front Immunol. 2017; 8: 65, doi:https://doi.org/10.3389/fimmu.2017.00065
- ^ Christoph Thomas and Robert Tampé, Structure of the TAPBPR−MHC I complex defines the mechanism of peptide loading and editing, Science, 2017, doi: https://doi.org/10.1126/science.aao6001
- ^ Calis JJA, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, et al. (2013) Properties of MHC Class I Presented Peptides That Enhance Immunogenicity. PLoS Comput Biol 9(10): e1003266. https://doi.org/10.1371/journal.pcbi.1003266.

