User:Joilybbi/5-HT3 antagonists drug discovery and development
5-HT3 receptor antagonists or serotonin receptor antagonists were first introduced in the early 1990s, and they have become the most widely used antiemetic drugs in chemotherapy.[1] They have also been proven safe and effective for treatment of postoperative nausea and vomiting.[2] Serotonin (5-HT) is found widely distributed throughout the gut and the central nervous system. In the gut, 5-HT is found mostly in mucosal enterochromaffin cells. Enterochromaffin cells are sensory transducers that release 5-HT to activate intrinsic (via 5-HT1P and 5-HT4 receptors) and extrinsic (via 5-HT3 receptors) primary afferent nerves.[3] Chemotherapeutic drugs for malignant disorders that cause vomiting have been found to cause release of large amounts of serotonin from enterochromaffin cells in the gut, serotonin acts on 5-HT3 receptors in the gut and brain stem. [3]
History
[edit]The history of 5-HT3 receptor antagonists began in 1957, when Gaddum and Picarelli proposed the existence of two 5-HT-receptor subtypes (M and D receptors) in a pioneering paper. The 5-HT3 receptor corresponds to the M receptor.[4] In the 1970s John Fozard proved, that metoclopramide and cocaine were weak 5-HT3 (5-HT-M) receptor antagonists and later synthesized MDL 72222, the first potent and truly selective 5-HT3 receptor antagonist, after teaming up with Marurice Gittos.[5][6] The antiemetic effects of metoclopramide where found to be partially because of the serotonin antagonism.[2] While Fozard was investigating cocaine analogues, workers at Sandoz identified the potent, selective 5-HT3 receptor antagonist ICS 205-930 from which the first marketed selective 5-HT3 receptor antagonists ondansetron and granisetron were developed, and approved in 1991 and 1993 respectively.[5][7] Several compounds related to MDL 72222 were synthesized which eventually resulted in approval of tropisetron in 1994 and dolasetron in 1997.[7]A new and improved 5-HT3 receptor antagonist, named palonosetron, was approved in 2003.[7] The development of selective 5-HT3 receptor antagonists was a dramatic improvement in the treatment of nausea and vomiting.[2] Ondansetron, granisetron, dolasetron and palonosetron are currently approved in the United States, and form the cornerstone of therapy for the control of acute emesis with chemotherapy agents with moderate to high emetogenic potential.[8]
Mechanism of action
[edit]The 5-HT3 receptors are present in several critical sites involved in emesis, including vagal affarents, thesolitary tract nucleus (STN), and the area postrema itself. Serotonin is released by the enterochromaffin cells of the small intestine in response to chemotherapeutic agents and may stimulate vagal affarents (via 5-HT3 receptors) to initiate the vomiting reflex. The 5-HT3 receptor antagonists suppress vomiting and nausea by inhibiting serotonin binding to the 5-HT3 receptors. The highest concentration of 5-HT3 receptors in the central nervous system (CNS) are found in the STN and chemoreceptor trigger zone (CTZ), and 5-HT3 antagonists may also suppress vomiting and nausea by acting at these sites.[1]
When patients undergo chemotherapy, serotonin is released from enterochromaffin cells by the cytotoxicity, the selective 5-HT3 receptor antagonists prevent the ability of serotonin to activate and sensitize gastrointestinal vagal-nerve terminals to other emetogenic substances released.[9]
The 5-HT3 receptor
[edit]The 5-HT3 (5-HT3) receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT (serotonin) receptors which are G protein-coupled receptors.[4][10][11] This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.[4] The rapidly activating, desensitizing, inward current is predominantly carried by sodium and potassium ions.[10] 5-HT3 receptors have a negligible permeability to anions.[4]
The 5-HT3 receptor consists of five subunits that may be the same (homopentameric 5-HT3A receptors) or different (heteropentameric receptors, usually comprising of 5-HT3A and 5-HT3B receptor subunits).[4][10][12]

The subunits surround a centralion channel in a pseudo-symmetric manner (Fig.1). Each subunit comprises an extracellular N-terminal domain, four transmembrane domains (M1-M4) connected by intracellular (M1-M2 and M3-M4) and extracellular loops (M2-M3) and anextracellular C-terminus (Fig.1).[4] The extracellular domain is the site of action of agonists and competitive antagonists because of ligand binding and the transmembrane domain controls the movement of ions across the cell membrane.[10] The human subunits 5-HT3A and 5-HT3B have been isolated and as well as sharing 41% amino acid sequence identity the location of their genes are in close proximity on the long arm of chromosome 11. The 5-HT3C, 5-HT3D and 5-HT3E subunits have not been isolated.[4]
Genes that code for the subunits of the 5-HT3 receptor have been identified. HTR3A and HTR3B for the 5-HT3A and 5-HT3B subunits and in addition HTR3C, HTR3D and HTR3E genes encoding 5-HT3C, 5-HT3D and 5-HT3E subunits. The latter three tend to show peripherally restricted pattern of expression, with high levels in the gut. In human duodenum and stomach, for example, 5-HT3C and 5-HT3E mRNA might be greater than for 5-HT3A and 5-HT3B. There is some evidence to suggest that the 5-HT3 receptor subunits are an important contribution to the effectiveness of these compounds.[10] In patients treated with chemotherapeutic drugs, certain polymorphism of the HTR3B gene could predict successful antiemetic treatment. This could indicate that the 5-HT3B receptor subunit could be used as biomarker of antiemetic drug efficacy. HTR3C and HTR3E do not seem to form functional homomeric channels, but when co-expressed with HTR3A they form heteromeric complex with decreased or increased 5-HT efficacies. The pathophysiological role for these additional subunits has yet to be identified.[9]
5-HT3 antagonists drug design
[edit]The 5-HT3 receptor ligand binding site
[edit]Experiments have shown evidence that the ligand-binding site is located at the interface of two adjacent subunits.[13] The ligand binding site is formed by three loops (A-C) from the principal ligand binding subunit (principal face) and three β-strands (D-F) from the adjacent subunit (complementary face). [4][11]The amino acid residue E129 on loop A faces into the binding pocket and forms a critical hydrogen bond with the hydroxyl group of 5-HT. Loop B contains W183, a critical tryptophan ligand binding residue that contributes to a cation-π interaction between the pi electron density of tryptophan and the primary amine of 5-HT. Loop C residues have been considered as candidates for the differing pharmacology of rodent and human 5-HT3 receptors because of their divergence between species. The most important aromatic residue within loop C is probably Y234 that lies opposite to the loop B tryptophan in the ligand binding pocket and is involved in ligand binding. Loops D and F are in fact β-strands not loops. W90 in loop D is critical for ligand binding and antagonists may directly contact R92. The azabicyclic ring of the competitive antagonist granisetron is located close to R92 and the aromatic rings lie close to W90. Loop E residues Y143, G148, E149, V150, Q151, N152, Y153 and K154 may be important for granisetron binding. The structure of loop F has yet to be clarified but W195 and D204 seem to be critical for ligand binding. [4] The results of several studies suggest that the orientation of granisetron (a 5-HT3 receptor antagonist )in the 5-HT3 receptor binding pocket is with its aromatic rings between Trp-183 and Tyr-234 and its azabicyclic ring between Trp-90 and Phe-226.(Fig.2)[10]
| 5-HT3 receptor antagonists | Binding affinity (Kd, Ki, K50) | Species |
|---|---|---|
| Tropisetron | 11nM | Human |
| Granisetron | 1,44 nM | Human |
| Ondansetron | 4,9 nM | Human |
| Palonosetron | 31,6 nM | Rat cerebral cortex, Rabbit ileal myenteric plexus, Guinea-pig ileal plexus |
| Dolasetron | 20,03 nM | NG 108-15 |
| Metocclopromide (non-selective) | 355 nM | Human |
| Cocaine | 2,45-83 nM | Rat-Rabbit |
The 5-HT3 receptor antagonists structure
[edit]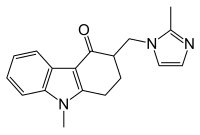
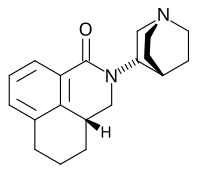
Chemical structures of the first generation 5-HT3 receptor antagonists can be categorized to three main classes[2]
1) Carbazole derivatives (ondansetron)
2) Indazoles (Granisetron)
3) Indoles(Tropisetron and Dolasetron)
The difference in structure of 5-HT3 receptor antagonists may contribute to the differences in pharmacological properties, like selectivity and binding affinity.[2]
5-HT3 receptor antagonists all have a basic amine, a rigid aromatic or heteroaromatic ring system and a carbonyl group (or isosteric equivalent) that is coplanar to the aromatic system, and there are slightly longer distances between the amine group and the aromatic when compared to the pharmacophore of 5-HT3 agonists.[10]
The first-generation 5-HT3 receptor antagonist (ondansetron, dolasetron, granisetron, and tropisetron) have been the most important drugs in antiemetic therapy for emetogenic chemotherapy. They are especially effective in treating acute emesis, occurring in the first 24 hours following chemotherapy.[8] A newer drug palonosetron is a pharmacologically distinct and highly selective, second generation 5-HT3 receptor antagonist.[14] Palonosetron has two stereogenic centers and exists as four stereoisomers.[14] Palonosetron has longer half-life (40h) and greater receptor binding affinity (>30 fold; when compared to first generation antagonists).[8]
The 5-HT3 receptor antagonists pharmacophore
[edit]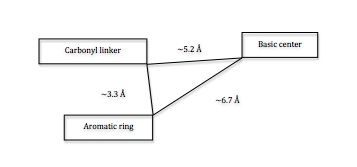
The pharmacophore of 5-HT3receptors consists of three components: a carbonyl-containing linking moiety, aromatic/heteroaromatic ring, and a basic center. The carbonyl group is coplanar to the aromatic ring. 5-HT3 receptor antagonists are more likely to bind in their protonated form.[15] Docking of a range of antagonists into a homology model of the 5-HT3 receptor binding site shows a reasonably good agreement with the pharmacophore model and supports the observed differences between species. Studies of granisetron in the binding pocket revealed that the aromatic rings of granisetron lie between W183 and Y234 and the azabiciclic ring between W90 and F226. In this study another energetically favorable location of granisetron was identified, closer to the membrane, on a position that could be a part of a binding/unbinding pathway for the ligand. A similarly located alternative binding site for granisetron has since been identified in another study of the 5-HT3 receptor.[10]
Structure-activity relationship (SAR)
[edit]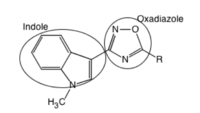
5-HT3 receptor antagonists share the same pharmacophore.[10] An aromatic moiety (preferably indole), a linking acyl group capable of hydrogen bonding interactions, and a basic amine (nitrogen) can be regarded as the key pharmacophoric elements of the known 5-HT3receptor antagonists. There are steric limitations of the aromatic binding site and although two hydrogen-bonding interactions are possible on the heterocyclic linking group (oxadiazole capable of accepting two hydrogen bonds), only one is essential for high affinity. An optimal environment of the basic nitrogen is when its constrained within an azabicyclin system with the highes affinity observed for systems with nitrogen at the bridgehead position and secondary amines being more potent.[16] The 5-HT3 receptor can only accommodate small substituents on the charged amine, a methyl group being optimal. [10] The optimal distance between the aromatic binding site and the basic amine is 8,4-8,9 Å and it is best if a two-carbon linkage separates the oxadiazole and the nitrogen. An increasing substitution of R increases affinity.[16] The most potent antagonists of 5-HT3 receptors have a 6-membered aromatic ring, and they usually have 6,5 heterocyclic rings.[10] No correlation has been found between the lipophilicity of compounds and the 5-HT3 receptor affinities.[17] Since most of the known 5-HT3 antagonists are ester or amide derivatives they are potentially susceptible to hydrolysis, which could be avoided by incorporating H-bond acceptors within a 5-membered heteroaromatic ring. [16]
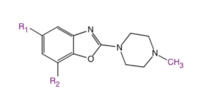
Structure-activity relationship (SAR) studies of LGIC receptor ligands are valuable to investigate their structure and function. An antagonist-like molecule with low intrinsic activity (ia) decreases the frequency of channel-opening and the permeability of ions. Small lipophilic C5 (R1) (see fig. 7) substituents afford compounds with potent antagonism which indicates that the C5 substituent may fit in a narrow, hydrophobic groove of the binding region in the receptor. It seems that the amino acid residues that interact with the C7 (R2) substituents have little to do with ligand binding but play a big role in ion channel gating. Sterically bulky substituents show a greater interaction with the gating amino acid residues and favor the open conformation af the ion channel because of sterical repulsion.[18]
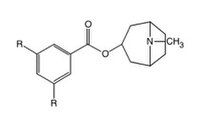
Ondansetron is a racemate but the stereochemistry of the asymmetric carbon atom is not an important factor in the 5-HT3 receptor interaction. Annelation of the 1,7-positions of the indole nucleus of ondansetron results in increased affinity for the receptor.[19]
A methyl group appears to be as effective functionally as a chlorine in the R position (see fig. 8). The carbonyl group is responsible for a strong interaction with the receptor and contributes significantly to the binding process. This carbonyl group is completely coplanar with the adjacent aromatic ring, indicating that the receptor-bound conformation corresponds to one of the most stable conformations of this group in the flexible compounds.[15]
Comparative pharmacology of 5-HT3 antagonists
[edit]Despite that the 5-HT3 receptor antagonist share their mechanism of action, they have different chemical structures and exhibit differences in affinity for the receptor, dose response and duration of effect. Also they are metabolized in different ways, that is different components of the cytochrome P450 (CYP) system are predominate in the metabolism of the antagonists.[2] The 5-HT3 receptor antagonist have similar activity. However patients who are resistant to one antagonist might benefit from another, possibly because the drugs are metabolized differently. A correlation is between the number of active CYP 2D6 alleles and the number of vomiting episodes by patients who receive treatment with cisplatin and ondansetron or tropisetron. Patients with multiple alleles tend to be unresponsive to the antiemetic drug and vice versa.[9]
| Drug | Chemical nature |
Receptor antagonists | T1/2 (h) | Metabolism | Dose |
|---|---|---|---|---|---|
| Ondansetron | Carbazole derivative | 5-HT3 receptor antagonist and weak 5-HT4 antagonist | 3.9 hours | CYP1A1/2, CYP2D6, CYP 3A3/4/5 | 0.15mg/kg |
| Granisetron | Indazole | 5-HT3 receptor antagonist | 9-11.6 hours | CYP3A3/4/5 | 10µg/kg |
| Dolasetron | Indole moiety | 5-HT3 receptor antagonist | 7-9 hours | CYP 3A3/4/5, CYP2D6 | 0.6-3 mg/kg |
| Palonosetron | Isoquinoline | 5-HT3 receptor antagonist; highest affinity for 5-HT3 receptor in this class | 40 hours | CYP1A2, CYP2D6, CYP3A3/4/5[20] | 0.25 mg/kg |
| Ramosetron | Benzidazolyl derivative | 5-HT3 receptor antagonist | 5.8 hours | 300 µg/kg | |
| Tropisetron [2] | Indole | 5-HT3 receptor antagonist | 5.6 hours | CYP 3A3/4/5, CYP2D6 | 200 µg/kg |
References
[edit]- ^ a b c Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (2006), Goddman & Gilman's The Pharmacological Basis of Therapeutics, New York: McGraw-Hill, pp. 1000–1003, ISBN 0-07-1442280-3
{{citation}}: Check|isbn=value: length (help) - ^ a b c d e f g Gan, Tong J. (2005), "Receptor Antagonists for Postoperative Nausea and Vomiting-Are They All the Same?", CNS Drugs, 19 (3): 225–238
- ^ a b kamm, M.A (2001), "Review article:the complexity of drug development for irritable bowel syndrome", Alimentari Pharmacology & Therapeutics, 16 (3): 343–351
- ^ a b c d e f g h i Barnes, Nicholas M.; Lummis, S.C.R; Peters, John (2008), "5-HT3 Receptors - the relationship between structure and function", Neuropharmacology, doi:10.1016/j.neuropharm.2008.08.003
- ^ a b King, Frank D.; Jones, Brian J.; Sanger, Gareth J. (1993), 5-Hydroxytryptamine-3 Receptor Antagonists, CRC Press, pp. 2–3, ISBN 0849354633, 9780849354632
{{citation}}: Check|isbn=value: invalid character (help) - ^ Galvan, M.; Gittos, M.; Fatmi, M. (October 1996), "DISCOVERY OF 5-HT3 RECEPTOR ANTAGONISTS AND DOLASETRON MESILATE", EJHP journal: 10–11
{{citation}}: Unknown parameter|Issue=ignored (|issue=suggested) (help)CS1 maint: date and year (link) - ^ a b c Billio, A.; Clarke, M.; Morello, E. (2006), "Comparison of clinical efficacy of serotonin receptor antagonists in highly emetogenic chemotherapy", Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD006272
{{citation}}: Unknown parameter|Issue=ignored (|issue=suggested) (help) - ^ a b c Oo, Thein H.; Hesketch, Paul J. (April 2005), "Drug Insight: new antiemetics in the management of chemotherapy-induced nausea and vomiting", Nature Clinical Practice Oncology, 2 (4): 198–201
{{citation}}: CS1 maint: date and year (link) - ^ a b c Sanger, Gareth J. (August 2008), "5- Hydroxytryptamine and the gastrointestinal tract: where next?", Trends in Pharmacological Sciences, 29: 465–471
{{citation}}: CS1 maint: date and year (link) - ^ a b c d e f g h i j k l Thompson, A.J; Lummis, S.C.R (2006), "5-HT3 Receptors", Current Pharmaceutical Design: 3615–3630
{{citation}}: Text "number 28" ignored (help); Text "volume 12" ignored (help) - ^ a b Reeves; lummis (January 2002), Molecular Membrane Biology, 19 (1): 11–26 http://www.ingentaconnect.com/content/apl/tmmb/2002/00000019/00000001/art00002=Citation
{{citation}}: Missing or empty|title=(help); Text "first D.C." ignored (help); Text "first2 S.C.R." ignored (help); Text "title The molecular basis of structure and function of the 5-HT3 receptor:a model ligand-gated ion channel (review)" ignored (help)CS1 maint: date and year (link) - ^ Boess, Frank G.; Beroukhim, Rameen; Martin, Ian L. (1995), "Ultrastructure of the 5-Hydroxytryptamine3 Receptor", Journal of Neurochemistry, 64: 1401–1405
- ^ Zhu, Li-Ping; Ye, De-Yong; Tang, Yun (June 2006), "Structure-Based 3D-QSAR studies on thiazoles as 5-HT3 receptor antagonist", Journal of Molecular Modeling, 13 (1): 121–131
{{citation}}: CS1 maint: date and year (link) - ^ a b Tian, Kan; Chen, Hongii; Tang, Xingguo; Hu, Zhide (September 2006), "Enantioseperation of palonosetron hydrochloride by micellar electrokinetic chromatography with sodium cholate as chiral selector", Journal of Chromatography, 1132: 333–336
{{citation}}: CS1 maint: date and year (link) - ^ a b Hibert, Marcel F.; Hoffmann, Remy; Miller, Robert C.; Carr, Albert A. (June 1990), "Conformation-Activity Relationship Study of 5-HT3 Receptor Antagonists and Definition of a Model for This Receptor Site", Journal of medicinal Chemistry, 33: 1594–1600
{{citation}}: Unknown parameter|Issue=ignored (|issue=suggested) (help)CS1 maint: date and year (link) - ^ a b c Baker, C.J; Kneen, C.; Moseley, J.; Saunders, J.; Seward, E.M.; Stevenson, G.; Beer, M.; Waitling, K. (January 1991), "Novel 5-HT3 Antagonists. Indole Oxidiazoles", Journal of Medicinal Chemistry, 34 (1): 140–151
{{citation}}: CS1 maint: date and year (link) - ^ Cappelli, Andrea; Donati, Anzini; Vomero, Salvatore; De Benedetti, Pier G.; Langer, Thierry (April 1996), "Molecular Structure and Dynamics of Some Potent 5-HT3 Receptor Antagonists. Insight into the Interaction with the Receptor", Bioorganic & Medicinal Chemistry, 4 (8): 1255–1269
{{citation}}: CS1 maint: date and year (link) - ^ Yoshida, S.; Watanabe, T.; Sato, Y. (2007), "Regulatory molecules for the 5-HT3 receptor ion channel gating system", Bioorganic & Medicinal Chemistry, 15 (10): 3515–3523
- ^ van Wijngaarden, I.; Hamminga, D.; van Hes, R.; Standaar, P.J; Tipker, J.; Tulp, Th. M.; Mol, F.; de Jonge, A. title = Development of High-Affinity 5-HT3 Receptor Antagonists. Structure-Affinity Relationships of Novel 1,7-Annelated Indole Derivatives. 1. (November 1993), Journal of Medicinal Chemistry, 36 (23): 3693–3699 http://www.ncbi.nlm.nih.gov/pubmed/8246239=Citation
{{citation}}: Missing or empty|title=(help); Missing pipe in:|first8=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Aapro, Matti (august 2005), "5-HT 3 -Receptor Antagonists in the Management of Nausea and Vomiting in Cancer and Cancer Treatment", Oncology, 69: 97–109
{{citation}}: Check date values in:|date=(help)CS1 maint: date and year (link)
