User:JamesFConley/EIF4A1
Eukaryotic initiation factor 4A-I is a 46 kDa cytosolic protein that in humans is encoded by the EIF4A1 gene, which is located on chromosome 17.[1][2][3] It is the most prevalent member of the eIF4A family of ATP-dependant RNA helicases, and plays a critical role in the initiation of cap-dependent eukaryotic protein translation as a component of the eIF4F translation initiation complex.[4] eIF4a1 unwinds the secondary structure of RNA within the 5'-UTR of mRNA, a critical step necessary for the recruitment of the 43S preinitiation complex, and thus the translation of protein in eukaryotes.[4] It was first characterized in 1982 by Grifo, et al., who purified it from rabbit reticulocyte lysate.[5]
Background
[edit]The regulation of the translation of mRNA transcripts into protein is one of the best ways that a cell can alter its response to its environment, as changes to the transcription of genes often takes considerably more time to be enacted. Protein translation can be broken into four phases: activation, initiation, elongation, and termination. Of these steps, initiation is the one for which cells have the most control. It is the rate limiting step of protein synthesis, controlled by a myriad of proteins known as the eukaryotic initiation factors, or eIFs. Relative abundance of these factors or their relative individual activities afford eukaryotic cells broad control over the rate of initiation and thus protein synthesis. eIFs are regulated under well known intracellular signalling pathways, such as the PI3K/AKT/mTOR pathway, however other biochemical layers of regulation, such as the complexity of RNA secondary structure in the 5′-UTR, are becoming evident with further research.[4]
The eIF4A subfamily in mammals is made up of three paralogs, eIF4A1, eIF4A2, and eIF4A3.[6] eIF4A1 and eIF4A2 share 90% sequence similarity, and are both cytoplasmic proteins, while eIF4A3 is localized to the nucleus, and shares only 60% homology.[6] Historically, eIF4A1 and eIF4A2 were considered interchangeable, due to this being observed in in vitro experiments, but further investigation has shown that eIF4A1 is more prevalent in dividing cells while eIF4A2 is more abundant in non-dividing cells, and furthermore, more recent evidence suggests that they might have functionally distinct roles in vivo.[4][6]
Structure
[edit]eIF4A1 is a member of the DEAD box family of RNA helicases.[7] RNA helicases are enzymes that use the energy released from the hydrolysis of ATP to manipulate the secondary structure of RNA, and the DEAD box family is the largest family of RNA helicases.[7] The name "DEAD box" refers the key D-E-A-D amino acid sequence on motif II of the helicase that participates in nucleoside triphosphate binding (in the instance of eIF4A1, ATP). Other conserved motifs, shared by all eIF4A family proteins, are the Q, I, Ia, Ib, III, IV, V and VI motifs. Motifs Ia, Ib, IV and V bind RNA, motifs I, II, and III mediate RNA-dependent ATPase activity, and motif VI, is required for both RNA binding and ATP hydrolysis.[6]

The DEAD box family is marked by a structurally highly conserved helicase core consisting of two RecA-like domains joined by a flexible hinge region around which the protein can open and close upon hydrolysis of ATP.[9][6][10] The cleft formed between these two domains forms the ATP-binding pocket.[7] The RNA molecule binds opposite to this binding pocket, stretching across each of the domains.[7] This core is flanked by variable auxiliary domains, which confer the unique function of each RNA helicase to them partly by allowing for specific binding to accessory proteins.[7]
Function
[edit]eIF4A1 is an ATP-dependent RNA helicase,[11] however the exact nature of its dependence on ATP for its function is still debated.[6] Although after ATP binding, the subsequent hydrolysis induces conformational changes in eIF4A1, other DEAD-box RNA helicases have been shown to possess helicase activity in the presence of nonhydrolyzable analogues of ATP, suggesting that binding, and not hydrolysis, is the more important element in regulating activity.[6]
eIF4A1 is a component of the eIF4F translation initiation complex, along with eIF4E, the 5'-terminal cap binding protein, and eIF4G, the scaffold protein that holds eIF4A and eIF4E together.[6] The eIF4F complex is accompanied by the accessory proteins eIF4B and eIF4H, both of which can enhance the activity of eIF4A1. After mRNA is transcribed from DNA and translocated to the cytoplasm, and the cytosolic PABP is bound to the Poly(A)-tail of the nascent mRNA, its 5'-cap will bind to eIF4E and PABP will bind to eIF4G.[4] eIF4A1 will then unwind the RNA secondary structure from 5' to 3' as the 43S PIC is recruited to the eIF4F complex.[4] The 43S PIC will scan the unwound mRNA from 5' to 3' as well, until it reaches the AUG start codon, whereupon the 60S ribosomal subunit will be recruited to begin the process of elongation.[4]
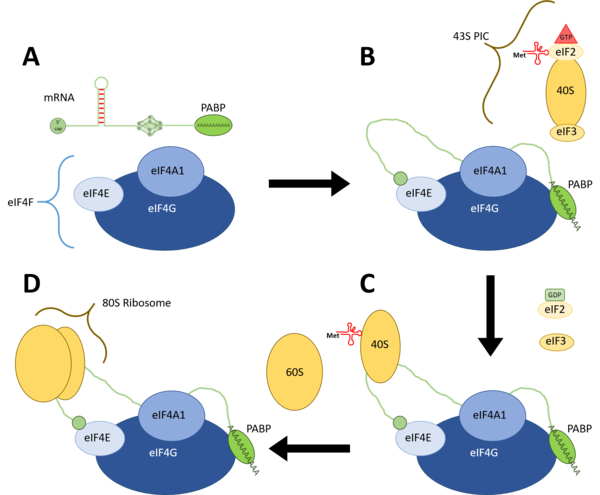
(B) eIF4A1 unwinding of mRNA secondary structure and recruitment of the 43S PIC.
(C) 40S ribosomal subunit scanning the 5'-UTR of the mRNA transcript for a start codon.
(D) Recruitment of the 60S ribosomal subunit and the beginning of elongation.
Regulation
[edit]The transcription of eIF4A1 is driven by the transcription factor MYC.[4] On its own, the helicase activity of eIF4A1 is poor, however this feature imposes a practical restraint on eIF4A1, as nonspecific, "unintended," helicase activity in the cell would be detrimental to the function of certain endogenous, necessary RNA structures.[6] Its effectiveness considerably improves in the presence of eIF4B and eIF4H, binding partners that modulate its activity. When eIF4B binds to eIF4A1, the helicase activity of eIF4A1 is increased over 100-fold, but when eIF4H binds instead, the increase is not nearly as great, suggesting different relative concentrations of these accessory proteins may confer a further level of regulation of the efficiency of eIF4A1.[6]
Conversely, eIF4A1 activity is suppressed when it is bound to PDCD4, a tumor suppressor itself modulated by mTOR and miR-21.[4] PCDC4 is typically localized to the nucleus in healthy cells, however, under carcinogenic conditions, it translocates to the nucleus and two separate eIF4A1 molecules will bind to it, inhibiting the ability of eIF4A1 to bind to RNA by locking the molecules into their inactive conformation, thereby preventing binding to eIF4G.[12][7]
Role in Disease
[edit]Cancer
[edit]Translational dysregulation is a hallmark of malignant transformation of cancer cells. Cancer cells in growing tumors become "addicted" to heightened levels of protein translation, and particularly dependent on up-regulated translation of pro-oncogenic mRNAs. These pro-oncogenic mRNAs have characteristically longer 5'-UTRs with more complex secondary structures, and up-regulation of eIF4A1 has been implicated in several human cancers (See Table).[4][13][14] Given the general trend of eIF4A1 overexpression driving cancer, there is interest in developing inhibitors for the enzyme. Several natural compounds have been identified as candidate inhibitors for development, though they inhibit both eIF4A1 and eIF4A2 non-specifically.[4] These include hippuristanol, silvestrol and pateamine A, among others.[4] Silvestrol, in particular is a rocaglate derivative, and this class of compounds could be viable eIF4A inhibitors.[15]
| Cancer Type | eIF4A1 Dysregulation/Association |
|---|---|
| Hepatocellular carcinoma | Overexpression[13] |
| Melanoma | Overexpression[13] |
| Non-small cell lung carcinoma (NSCLC) | Expression associated with metastasis[4] |
| Endometrial cancer | Overexpression in atypical hyperplasia[4] |
| Cervical Cancer | Overexpression; decreased expression after brachytherapy associated with better outcome[4] |
| Breast Cancer | Expression associated with poor outcome in estrogen receptor negative disease[4] |
Viral Infections
[edit]Viruses rely on hijacking the cellular machinery of the cells they infect to create their own viral proteins and allow them to continue infecting new cells. Their ability to manipulate eIFs like eIF4A1, therefore, considerably impacts their virulence. For instance, cytomegalovirus relies on eIF4A to drive its protein synthesis. The viral protein pUL69 stabilizes the formation of eIF4F, through binding to eIF4A, a process by which eIF4E is prevented from dissociating from the eIF4F complex.[10] eIF4E, thus, is no longer able to be sequestered by its negative regulator, 4EBP.[10] Furthermore, cytomegalovirus stimulates the synthesis of all elements of the eIF4F complex in order to drive protein synthesis.[10] Other viruses, like Cotesia plutellae bracovirus (CpBV), that favor cap-independent translation, will take advantage of eIF4A1 in the reverse context, by sequestering eIF4A1 away from the eIF4F complex with viral binding partners, in this case a protein called CpBV15β, thus inhibiting endogenous cap-dependent mRNA translation and favoring viral protein translation.[10] The compounds mentioned in the above section about cancer, hippuristanol, silvestrol, pateamine A, rocaglate derivatives, etc., could also be applied as putative viral inhibitors.[4][15]
References
[edit]- ^ Kim NS, Kato T, Abe N, Kato S (April 1993). "Nucleotide sequence of human cDNA encoding eukaryotic initiation factor 4AI". Nucleic Acids Research. 21 (8): 2012. doi:10.1093/nar/21.8.2012. PMC 309447. PMID 8493113.
- ^ Jones E, Quinn CM, See CG, Montgomery DS, Ford MJ, Kölble K, et al. (October 1998). "The linked human elongation initiation factor 4A1 (EIF4A1) and CD68 genes map to chromosome 17p13". Genomics. 53 (2): 248–50. doi:10.1006/geno.1998.5515. PMID 9790779.
- ^ "Entrez Gene: EIF4A1 eukaryotic translation initiation factor 4A, isoform 1".
- ^ a b c d e f g h i j k l m n o p q Raza F, Waldron JA, Quesne JL (December 2015). "Translational dysregulation in cancer: eIF4A isoforms and sequence determinants of eIF4A dependence". Biochemical Society Transactions. 43 (6): 1227–33. doi:10.1042/BST20150163. PMID 26614665.
- ^ Grifo JA, Tahara SM, Leis JP, Morgan MA, Shatkin AJ, Merrick WC (May 1982). "Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA". The Journal of Biological Chemistry. 257 (9): 5246–52. PMID 7068683.
- ^ a b c d e f g h i j k Lu WT, Wilczynska A, Smith E, Bushell M (February 2014). "The diverse roles of the eIF4A family: you are the company you keep". Biochemical Society Transactions. 42 (1): 166–72. doi:10.1042/BST20130161. PMID 24450646.
- ^ a b c d e f Linder P, Jankowsky E (July 2011). "From unwinding to clamping - the DEAD box RNA helicase family". Nature Reviews. Molecular Cell Biology. 12 (8): 505–16. doi:10.1038/nrm3154. PMID 21779027.
- ^ "EIF4A1 - Eukaryotic initiation factor 4A-I - Homo sapiens (Human) - EIF4A1 gene & protein". www.uniprot.org.
- ^ Sharma D, Jankowsky E (20 July 2014). "The Ded1/DDX3 subfamily of DEAD-box RNA helicases". Critical Reviews in Biochemistry and Molecular Biology. 49 (4): 343–60. doi:10.3109/10409238.2014.931339. PMID 25039764.
- ^ a b c d e Montero, Hilda; Pérez-Gil, Gustavo; Sampieri, Clara L. (22 February 2019). "Eukaryotic initiation factor 4A (eIF4A) during viral infections". Virus Genes. 55 (3): 267–273.
- ^ Shatsky IN, Dmitriev SE, Andreev DE, Terenin IM (1 March 2014). "Transcriptome-wide studies uncover the diversity of modes of mRNA recruitment to eukaryotic ribosomes". Critical Reviews in Biochemistry and Molecular Biology. 49 (2): 164–77. doi:10.3109/10409238.2014.887051. PMID 24520918.
- ^ "PDCD4 programmed cell death 4 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov.
- ^ a b c Ali MU, Ur Rahman MS, Jia Z, Jiang C (June 2017). "Eukaryotic translation initiation factors and cancer". Tumour Biology. 39 (6): 1010428317709805. doi:10.1177/1010428317709805. PMID 28653885.
- ^ Abdelhaleem M (July 2004). "Do human RNA helicases have a role in cancer?". Biochimica et Biophysica Acta. 1704 (1): 37–46. doi:10.1016/j.bbcan.2004.05.001. PMID 15238243.
- ^ a b Pan, Li; Woodard, John L.; Lucas, David M.; Fuchs, James R.; Kinghorn, A. Douglas (2 May 2014). "Rocaglamide, Silvestrol and Structurally Related Bioactive Compounds from Aglaia Species". Natural Product Reports. 31 (7): 924–939.
Further reading
[edit]- Reddy NS, Roth WW, Bragg PW, Wahba AJ (October 1988). "Isolation and mapping of a gene for protein synthesis initiation factor 4A and its expression during differentiation of murine erythroleukemia cells". Gene. 70 (2): 231–43. doi:10.1016/0378-1119(88)90195-3. PMID 3215517.
- Kukimoto I, Watanabe S, Taniguchi K, Ogata T, Yoshiike K, Kanda T (April 1997). "Characterization of the cloned promoter of the human initiation factor 4AI gene". Biochemical and Biophysical Research Communications. 233 (3): 844–7. doi:10.1006/bbrc.1997.6555. PMID 9168945.
- Imataka H, Sonenberg N (December 1997). "Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A". Molecular and Cellular Biology. 17 (12): 6940–7. doi:10.1128/mcb.17.12.6940. PMC 232551. PMID 9372926.
- Gradi A, Imataka H, Svitkin YV, Rom E, Raught B, Morino S, Sonenberg N (January 1998). "A novel functional human eukaryotic translation initiation factor 4G". Molecular and Cellular Biology. 18 (1): 334–42. doi:10.1128/mcb.18.1.334. PMC 121501. PMID 9418880.
- Craig AW, Haghighat A, Yu AT, Sonenberg N (April 1998). "Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation". Nature. 392 (6675): 520–3. Bibcode:1998Natur.392..520C. doi:10.1038/33198. PMID 9548260.
- Henis-Korenblit S, Strumpf NL, Goldstaub D, Kimchi A (January 2000). "A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation". Molecular and Cellular Biology. 20 (2): 496–506. doi:10.1128/MCB.20.2.496-506.2000. PMC 85113. PMID 10611228.
- Quinn CM, Wiles AP, El-Shanawany T, Catchpole I, Alnadaf T, Ford MJ, et al. (December 1999). "The human eukaryotic initiation factor 4AI gene (EIF4A1) contains multiple regulatory elements that direct high-level reporter gene expression in mammalian cell lines". Genomics. 62 (3): 468–76. doi:10.1006/geno.1999.6031. PMID 10644445.
- Cuesta R, Xi Q, Schneider RJ (July 2000). "Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F". The EMBO Journal. 19 (13): 3465–74. doi:10.1093/emboj/19.13.3465. PMC 313943. PMID 10880459.
- Mendell JT, Medghalchi SM, Lake RG, Noensie EN, Dietz HC (December 2000). "Novel Upf2p orthologues suggest a functional link between translation initiation and nonsense surveillance complexes". Molecular and Cellular Biology. 20 (23): 8944–57. doi:10.1128/MCB.20.23.8944-8957.2000. PMC 86549. PMID 11073994.
- Li W, Belsham GJ, Proud CG (August 2001). "Eukaryotic initiation factors 4A (eIF4A) and 4G (eIF4G) mutually interact in a 1:1 ratio in vivo". The Journal of Biological Chemistry. 276 (31): 29111–5. doi:10.1074/jbc.C100284200. PMID 11408474.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Du MX, Johnson RB, Sun XL, Staschke KA, Colacino J, Wang QM (April 2002). "Comparative characterization of two DEAD-box RNA helicases in superfamily II: human translation-initiation factor 4A and hepatitis C virus non-structural protein 3 (NS3) helicase". The Biochemical Journal. 363 (Pt 1): 147–55. doi:10.1042/0264-6021:3630147. PMC 1222461. PMID 11903057.
- Bohnsack MT, Regener K, Schwappach B, Saffrich R, Paraskeva E, Hartmann E, Görlich D (November 2002). "Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm". The EMBO Journal. 21 (22): 6205–15. doi:10.1093/emboj/cdf613. PMC 137205. PMID 12426392.
- Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH (May 2004). "A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A". Molecular and Cellular Biology. 24 (9): 3894–906. doi:10.1128/MCB.24.9.3894-3906.2004. PMC 387765. PMID 15082783.
- Mingot JM, Bohnsack MT, Jäkle U, Görlich D (August 2004). "Exportin 7 defines a novel general nuclear export pathway". The EMBO Journal. 23 (16): 3227–36. doi:10.1038/sj.emboj.7600338. PMC 514512. PMID 15282546.
- Hinton TM, Coldwell MJ, Carpenter GA, Morley SJ, Pain VM (January 2007). "Functional analysis of individual binding activities of the scaffold protein eIF4G". The Journal of Biological Chemistry. 282 (3): 1695–708. doi:10.1074/jbc.M602780200. PMID 17130132.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.