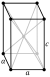User:Double sharp/Tin
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | silvery-white, β (beta); gray, α (alpha) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Sn) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 505.08 K (231.93 °C, 449.47 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2875 K (2602 °C, 4716 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | white (β): 7.289 g/cm3 gray (α): 5.770 g/cm3[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.99 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | white (β): 7.03 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | white (β): 296.1 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | white (β): 27.112 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: −4, +2, +4 −3,[4] −2,[5] −1,[6] 0,[7] +1,[8] +3[9] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 140 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 139±4 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 217 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | white (β): body-centered tetragonal (tI4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | white (β): a = 583.13 pm c = 318.11 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | gray (α): face-centered diamond-cubic (cF8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | gray (α): a = 648.96 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | white (β): 21.76×10−6/K (at 20 °C)[a] gray (α): 5.20×10−6/K (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 66.8 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 115 nΩ⋅m (at 0 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | white (β): paramagnetic gray (α): diamagnetic[10] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | white (β): +3.1×10−6 cm3/mol (298 K)[11] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 18 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 58 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2730 m/s (at r.t.) (rolled) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 50–440 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-31-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | protohistoric, around 35th century BC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Sn": from Latin stannum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of tin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tin is a chemical element with symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal in group 14 of the periodic table whose chemistry is intermediate between those of its vertical neighbours, germanium and lead. Tin is obtained chiefly from the mineral cassiterite, which contains tin dioxide, SnO2. Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, thanks to its magic number of protons. It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal, but at low temperatures it transforms into the less dense grey α-tin, which has the diamond cubic structure. Metallic tin is not easily oxidized in air or water at room temperature, though it does react with steam and with air on heating.
The first alloy used on a large scale was bronze, made of tin and copper, from as early as 3000 BC. After 600 BC, pure metallic tin was produced. Pewter, which is an alloy of 85–90% tin with the remainder commonly consisting of copper, antimony, and lead, was used for flatware from the Bronze Age until the 20th century. In modern times, tin is used in many alloys, most notably tin/lead soft solders, which are typically 60% or more tin and in the manufacture of transparent, electrically conducting films of indium tin oxide in optoelectronic applications. Another large application for tin is corrosion-resistant tin plating of steel. Because of the low toxicity of inorganic tin, tin-plated steel is widely used for food packaging as tin cans. However, some organotin compounds (compounds formed from tin and hydrocarbons) can be almost as toxic as cyanide.
Characteristics
[edit]Physical and atomic
[edit]A tin atom has fifty electrons, arranged in the electron configuration [Kr]4d105s25p2. Of these, four are valence electrons, occupying the 5s and 5p subshells. Like the other stable members of group 14, carbon, silicon, germanium, and lead, tin has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of xenon by forming sp3 hybrid orbitals, forming tetrahedral tetravalent SnX4 derivatives where the central tin atom shares an electron pair with each of the four atoms it is bonded to.[13] Nevertheless, tin also shows significant chemistry in the divalent state, in which only the 5p electrons are involved in bonding: the trend down the group favours the lower +2 oxidation state over the higher +4 for the heavier elements, as bond energy decreases with size from silicon to lead so that the energy needed to use the 5s electrons to form bonds is not compensated by the energy released in forming these bonds, particularly when highly electronegative substituents are involved.[14][15] The first four ionisation energies of tin are 708.4, 1411.4, 2942.2, and 3929.3 kJ/mol respectively, which are close to the values for lead due to the lanthanide contraction. These values are less than those for carbon, silicon, and germanium and accordingly tin is the first element in group 14 to show significant cationic chemstry. Tin has an electronegativity of about 1.96 on the Pauling scale; this is intermediate between the values for germanium (2.01) and lead(II) (1.87), greater than the value for silicon (1.90), and significantly less than the value for lead(IV) (2.33).[16]
Tin forms two allotropes
Isotopes
[edit]Chemistry
[edit]Hydrides and derivatives
[edit]Halides and derivatives
[edit]Oxides and hydroxides
[edit]Oxoacid derivatives
[edit]and are they really salts? really Sn is a lame metal chemically
Other inorganic compounds
[edit]Cluster compounds
[edit]Organotin compounds
[edit]Origin and occurrence
[edit]Etymology
[edit]History
[edit]Production
[edit]Applications
[edit]Biological effects
[edit]Bibliography
[edit]- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. ISBN 978-0-19-927029-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- King, R. Bruce (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. ISBN 0-471-18602-3.
References
[edit]- ^ "Standard Atomic Weights: Tin". CIAAW. 1983.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d e Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Sn(−3) has been observed in [Sn2]6−, e.g. in (Ba2)4+(Mg4)8+Sn4−(Sn2)6−Sn2− (with square (Sn2−)n sheets), see Papoian, Garegin A.; Hoffmann, Roald (2000). "Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks". Angew. Chem. Int. Ed. 2000 (39): 2408–2448. doi:10.1002/1521-3773(20000717)39:14<2408::aid-anie2408>3.0.co;2-u. PMID 10941096. Retrieved 2015-02-23.
- ^ Sn(−2) has been observed in SrSn; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2008). Lehrbuch der Anorganischen Chemie (in German) (102 ed.). Walter de Gruyter. p. 1007. ISBN 9783110206845.
- ^ Sn(−1) has been observed in CsSn; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2008). Lehrbuch der Anorganischen Chemie (in German) (102 ed.). Walter de Gruyter. p. 1007. ISBN 9783110206845.
- ^ "New Type of Zero-Valent Tin Compound". Chemistry Europe. 27 August 2016.
- ^ "HSn". NIST Chemistry WebBook. National Institute of Standards and Technology. Retrieved 23 January 2013.
- ^ "SnH3". NIST Chemistry WebBook. National Institure of Standards and Technology. Retrieved 23 January 2013.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ King, pp. xiii–xviii
- ^ Greenwood and Earnshaw, pp. 226–7
- ^ Kaupp, Martin (1 December 2006). "The role of radial nodes of atomic orbitals for chemical bonding and the periodic table" (PDF). Journal of Computational Chemistry. 28 (1): 320–25. doi:10.1002/jcc.20522. Retrieved 7 February 2018.
- ^ Greenwood and Earnshaw, pp. 371–2
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).