User:Double sharp/Template Periodic table
How I'd draw the table if writing about periodicity myself. I also advocate this as ideal general form (18 vs 32 column is cosmetics, to me they're the same thing), but that will take some time and advocacy. :-P
This is never ever ever going to appear on WP as the main form obviously unless the vast majority of the literature moves to it (which is so not happening anytime soon if ever, although one can dream).
I don't like having to cut out the f elements, but it appears necessary to have the whole thing be visible at once. Remember that the cells have to be wide enough to fit the element names! No, I don't think it creates anything different: a map of the United States is still a map of the United States even if Alaska and Hawaii are not put in their geographically correct location for space.
Light colours denote nonmetals; dark ones – metals. Defined by metallic bonding of all stable/metastable allotropes (so Sb counts as a metal, As does not). Why, yes, metallicity by this definition changes according to temperature and pressure: in the dead of winter Sn and Sb are nonmetals, at stupidly high pressures everything is a metal. (α-Ge is like α-Sn; β-Ge, stable above 11 GPa, is like β-Sn.) So what, given that state of matter changes too? (BTW, Si becomes a metal when it melts.) So both are tabulated at 30 °C and at 1 atm. Data is predicted for the elements not seen in enough quantities yet, and might change in the future as better calculations come along (not ruling it out for Cn and Og, maybe not even At; all three have changed before). This said, I start to wonder if it might not just be more plain pragmatic to just draw a diagonal step as close enough for most cases.
Solid borders denote elements that have survived since the Earth's formation: they're basically stable (or almost so, in the cases of Th and U; Bi radioactivity is not worth mentioning). Dashed ones are their intermediate decay products. (Plutonium is considered primordial here, because with its long half-life there should be some left; it's just hard to find for sure.) Dotted ones are synthetic; Nature doesn't give them to us on the planet, we made them ourselves.
| Period Group | Is | IIs | IIId | IVd | Vd | VId | VIId | VIIId | IXd | Xd | XId | XIId | IIIp | IVp | Vp | VIp | VIIp | VIIIp | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 1s |
|||||||||||||||||||
| 2 2s 2p |
|||||||||||||||||||
| 3 3s 3p |
|||||||||||||||||||
| 4 4s 3d 4p |
|||||||||||||||||||
| 5 5s 4d 5p |
|||||||||||||||||||
| 6 6s 4f 5d 6p |
4f | ||||||||||||||||||
| 7 7s 5f 6d 7p |
5f | ||||||||||||||||||
| IIIf | IVf | Vf | VIf | VIIf | VIIIf | IXf | Xf | XIf | XIIf | XIIIf | XIVf | XVf | XVIf | ||||||
| 4f | |||||||||||||||||||
| 5f | |||||||||||||||||||
1 (red)=Gas 3 (black)=Solid 80 (green)=Liquid Color of the atomic number shows state of matter (at 30 °C and 1 atm)
- Ca: 40.078 — Formal short value, rounded (no uncertainty)[2]
- Po: [209] — mass number of the most stable isotope
There are some names for rather homogeneous sets of elements, the following of which seem most useful to actually use in running text:
- Alkali metals: Li, Na, K, Rb, Cs, Fr
- Alkaline earth metals: Ca, Sr, Ba, Ra (many sources give Be and Mg as belonging to this category, but they are not alkaline)
- Chalcogens: O, S, Se, Te (Po is ambiguous)
- Halogens: F, Cl, Br, I (At is ambiguous)
- Noble gases: He, Ne, Ar, Kr, Xe, Rn (the last with some asterisks).
I do not really believe that Cn is a "noble liquid", sorry. The predictions seem to differ a lot.
There are of course other useful ones, like pnictogens (N, P, As, maybe Sb), crystallogens (C, Si, Ge, maybe Sn), platinum group metals (Ru, Rh, Pd, Os, Ir, Pt), coinage metals (Cu, Ag, Au although they are rather united mostly by being weirdly different from each other), rare earth elements, etc. etc. But these seem most useful. I'm not too fussed about all these categories overlapping since for me they were always only meant as grouping together like elements anyway. If an element is near the intersection of two groups, who am I to force it to declare only a single allegiance?
Rather than talking about rare earth elements, I'd simply talk about f elements and include group IIId as comparison.
Transitionness is rather a degree I think: scandium is a bit iffy as transition, titanium too (but less iffy), but vanadium first has multiple oxidation states of comparable stability. So I would probably speak of "transition" fairly loosely and not try to define it too well.
I would go extremely heavy on a drilling-down-to-first-principles approach (consistent with the Sc-Y-Lu + He-Be).
32 column (expanded to include projected period 8):
This is a "stricter" version, in which metallicity and phase at STP are ignored as they are about the simple substance rather than the element (and vary with conditions anyway), and in which occurrence and atomic weights are removed as Earth-centric (although the last is probably purism taken beyond the point of usefulness).
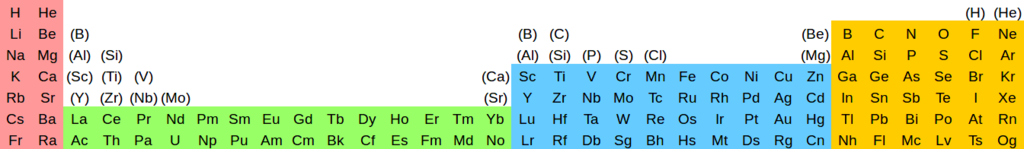
A version showing pseudohomologies:
- ^ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–291. doi:10.1515/pac-2015-0305.
- ^ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3). Table 2, 3 combined; uncertainty removed. doi:10.1515/pac-2015-0305.
