User:AKmicrobio/Bacteroides fragilis
Bacteroides fragilis
[edit]
| AKmicrobio/Bacteroides fragilis | |
|---|---|
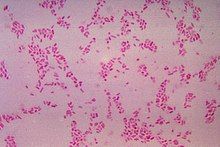
| |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacteroidota |
| Class: | Bacteroidia |
| Order: | Bacteroidales |
| Family: | Bacteroidaceae |
| Genus: | Bacteroides |
| Species: | B. fragilis
|
| Binomial name | |
| Bacteroides fragilis (Veillon and Zuber 1898) Castellani and Chalmers 1919 (Approved Lists 1980)
| |
Bacteroides fragilis is an anaerobic, Gram-negative, pleomorphic to rod-shaped bacterium. It is part of the normal microbiota of the human colon and is generally commensal,[1][2] but can cause infection if displaced into the bloodstream or surrounding tissue following surgery, disease, or trauma.[3]
Habitat
[edit]Bacteroides fragility resides in the human gastrointestinal tract and it essential to healthy gastrointestinal function such as mucosal immunity and host nutrition.[4] As a mesophile, optimal growth occurs at 37°C and a pH around 7.[5][4]
Morphology
[edit]Cells of B. fragilis are rod-shaped to pleomorphic with a cell size range of 0.5-1.5 x 1.0-6.0 μm.[4]B. fragilis is a Gram-negative bacterium and does not possess and flagella or cilia making it immotile. However, it does utilize peritrichous fimbriae for adhesion to other molecular structures. B. fragilis also utilizes a complex series of surface proteins, lipopolysaccharide chains, and outer membrane vesicles to help survive the volatile intestinal micro-environment.[6]
Metabolism & Mutualism in the Gut Microbiome
[edit]B. fragilis is a aerotolerant, anaerobic chemoorganotroph capable of fermenting a wide variety of glycans available in the human gut microenvironment including glucose, sucrose, & fructose. Can also catabolize a variety of biopolymers, polysaccharides, and glycoproteins into smaller molecules which can then be used and further broken down by other microbes. Also produces fatty acid as a byproduct of carbohydrate fermentation which can then be used as energy by the host.[6][4] It was discovered that Cytochrome bd oxidase is essential to oxygen consumption. This can allow other obligate anaerobes to survive in the now oxygen-reduced microenvironment. [7][6] Animals lacking gut bacteria require 30% more caloric intake to maintain body mass.[6]
Environment-Sensing Systems
[edit]The complex environmental-sensory system allows B. fragilis to survive/adapt in the ever-changing human gut microbiome. This system is composed of/designed to effectively handle:
Bacteriocins: B. fragilis intestinal isolates secrete high levels of bacteriocin proteins and are resistant to other bacteriocins secreted by other closely related isolates. This mechanism is believed to reduce the level of intra-specific competition.[4]
Bile salt resistance: Utilizes enzymes such as "bile salt hydrolase" to resist the degrading effects of bile salts. Detergent activity of bile salts can permeabilize bacterial membranes which can eventually lead to membrane collapse and/or cell damage.[4]
Oxidative Stress Response: Proteins such as catalase, superoxide dismutase, & alkyl hydroperoxide reductase protect the organism from harmful oxygen radicals. This permits growth in the presence of nanomolar concentrations of O2.[4]
Antibiotic Resistance
[edit]Member of the genus Bacteroides are characterized with having the highest numbers of antibiotic resistance mechanisms accompanied by the highest resistance rates amongst anaerobic bacteria. Species of the Bacteroidaceae have displayed increasing resistance to antimicrobial agents such as cefoxitin, clindamycin, metronidazole, carbapenems, & fluoroquinalones.[6][4]
Resistance Reservoirs: Bacteroides species accumulate a variety of antibiotic/antimicrobial resistance genes as they reside in the gastrointestinal tract. This allows the genetic transfer of these genes to other Bacteroides species and possibly other more virulent bacteria leading to an overall increase in multi-drug resistance. This is exacerbated by the tendency of resistance genes to be relatively stable even without the presence of the antibiotic.[6]
Epidemiology and Pathogenesis
[edit]The B. fragilis group is the most commonly isolated Bacteroidaceae in anaerobic infections, especially those that originate from the gastrointestinal microbiota. B. fragilis is the most prevalent organism in the B. fragilis group, accounting for 41% to 78% of the isolates of the group. These organisms are resistant to penicillin by virtue of production of beta-lactamase, and by other unknown factors.[8]
This group was formerly classified as subspecies of B. fragilis (i.e. B. f. ssp. fragilis, B. f. ssp. distasonis, B. f. ssp. ovatus, B. f. ssp. thetaiotaomicron, and B. f. ssp. vulgatus). They have been reclassified into distinct species on the basis of DNA homology studies.[9] B. fragilis (formerly known as B. f. ssp. fragilis) is often recovered from blood, pleural fluid, peritoneal fluid, wounds, and brain abscesses.
Although the B. fragilis group is the most common species found in clinical specimens, it is the least common Bacteroides present in fecal microbiota, comprising only 0.5% of the bacteria present in stool. Their pathogenicity partly results from their ability to produce capsular polysaccharide, which is protective against phagocytosis[6] and stimulates abscess formation.[3]
Bacteroides fragilis is involved in 90% of anaerobic peritoneal infections.[10] It also causes bacteremia[11] associated with intra-abdominal infections, peritonitis and abscesses following rupture of viscus, and subcutaneous abscesses or burns near the anus.[12] Though it is gram negative, it has an altered LPS and does not cause endotoxic shock. Untreated B. fragilis infections have a 60% mortality rate.[6]
See also
[edit]External links
[edit]- Bacteroides references in Baron's Medical Microbiology (online at the NCBI bookshelf).
- Type strain of Bacteroides fragilis at BacDive - the Bacterial Diversity Metadatabase
References
[edit]- ^ Kuwahara T, Yamashita A, Hirakawa H, et al. (October 2004). "Genomic analysis of Bacteroides fragilis reveals extensive DNA inversions regulating cell surface adaptation". Proc. Natl. Acad. Sci. U.S.A. 101 (41): 14919–24. Bibcode:2004PNAS..10114919K. doi:10.1073/pnas.0404172101. PMC 522005. PMID 15466707.
- ^ http://prod.hopkins-abxguide.org/pathogens/bacteria/anaerobic_gram-neg._bacilli/bacteroides_fragilis.html?contentInstanceId=255919[permanent dead link]
- ^ a b Levinson, W. (2010). Review of Medical Microbiology and Immunology (11th ed.).
- ^ a b c d e f g h Wexler, Hannah M. (2014), Rosenberg, Eugene; DeLong, Edward F.; Lory, Stephen; Stackebrandt, Erko (eds.), "The Genus Bacteroides", The Prokaryotes: Other Major Lineages of Bacteria and The Archaea, Berlin, Heidelberg: Springer, pp. 459–484, doi:10.1007/978-3-642-38954-2_129, ISBN 978-3-642-38954-2, retrieved 2021-11-13
- ^ "BacMap". bacmap.wishartlab.com. Retrieved 2021-11-13.
- ^ a b c d e f g h Wexler, Hannah M. (2007-10-01). "Bacteroides: the Good, the Bad, and the Nitty-Gritty". Clinical Microbiology Reviews. 20 (4): 593–621. doi:10.1128/CMR.00008-07. PMC 2176045. PMID 17934076.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Baughn, Anthony D.; Malamy, Michael H. (2004-01). "The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen". Nature. 427 (6973): 441–444. doi:10.1038/nature02285. ISSN 1476-4687.
{{cite journal}}: Check date values in:|date=(help) - ^ Snydman DR, Jacobus NV, McDermott LA, et al. (January 2010). "Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005–2007)". Clin. Infect. Dis. 50 (Suppl 1): S26–33. doi:10.1086/647940. PMID 20067390.
- ^ Baron EJ, Allen SD (June 1993). "Should clinical laboratories adopt new taxonomic changes? If so, when?". Clin. Infect. Dis. 16 (Suppl 4): S449–50. doi:10.1093/clinids/16.Supplement_4.S449. PMID 8324167.
- ^ Bacteroides infections at eMedicine
- ^ Brook I (June 2010). "The role of anaerobic bacteria in bacteremia". Anaerobe. 16 (3): 183–9. doi:10.1016/j.anaerobe.2009.12.001. PMID 20025984.
- ^ Brook I (October 2008). "Microbiology and management of abdominal infections". Dig. Dis. Sci. 53 (10): 2585–91. doi:10.1007/s10620-007-0194-6. PMID 18288616. S2CID 35945399.
