User:2000yearslater/Drugs and sexually transmitted diseases
Drugs and medications could be used to treat sexually transmitted diseases (STDs). STDs are infections that can be spread through sexual activities.[1] Leaving STDs untreated could lead to severe or lifelong complications. Hence, immediate treatment is crucial to prevent the further spread of STDs to sexual partners.[2] Comprehensive counseling intervention serves as the primary means for prevention against STDs. Since most STDs remain asymptomatic, people who are sexually active should be tested regularly by microscopic examinations and blood tests.[1][2]
Drugs and medications used to treat STDs are typically divided into two classes: antiviral medications for STDs caused by viruses and antibiotics for STDs caused by bacteria or parasites.[3] STDs caused by viruses cannot be cured. Nonetheless, medications can be used to alleviate the symptoms and lower the risk of transmitting viral infection.[4] For example, the highly active antiretroviral therapy (HAART) can be used to slow the progression of AIDS and reduce can reduce patients' mortality rate.[5] Vaccines are also highly effective in preventing human papillomavirus (HPV) and hepatitis B.[3] On the other hand, STDs caused by bacteria can be cured by immediate treatment. However, a full course of antibiotics should be taken to prevent antibiotic resistance.[4] The most common antibiotics prescribed are azithromycin, doxycycline, ciprofloxacin, and ceftriaxone.[6]
STDs caused by viruses
[edit]STDs can be caused by viral infections. Viral STDs with the highest prevalence include hepatitis B, acquired immunodeficiency syndrome (AIDS) and genital herpes.[7]
Hepatitis B
[edit]Hepatitis B is a liver infection caused by the hepatitis B virus (HBV). It is sexually transmitted and can be categorized into 2 types: acute infection and chronic infection.[7] A lot of people are asymptomatic during the initial infection.[8] For people who show symptoms, they include jaundice, fatigue, abdominal pain and poor appetite.[8]
Interferon injections
[edit]Interferon injections are used to treat chronic hepatitis B infections by inhibiting HBV viral replication. The injection contains an alpha interferon called interferon Alfa-2B which can induce an antiviral state in cells.[9] Subsequently, interferon Alfa-2B binds to cell surface receptors and activates gene expression in the cells, prompting the breakdown of viral RNAs and protecting the cells against viral infection.[9] After a 48-week course and receiving injection 3 times per week, the levels of HBV reduce greatly in more than 50% of the patients.[9][10]
Side effects of the injection include fever, fatigue and muscle pains. The injection can also reduce the levels of hemoglobin, white blood cells and platelets in some patients. Hence, patients with blood cell disorders such as sickle cell anemia are strongly advised not to take the interferon injections.[10]
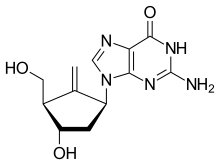
Entecavir
[edit]Entecavir (ETV) is an antiviral drug used to treat chronic hepatitis B infections. It is taken as a tablet or solution form orally. Entecavir is a nucleoside inhibitor that hinders the action of HBV polymerase, an enzyme responsible for HBV replication.[11]
Common side effects include headache, fatigue, dizziness and nausea.[12]
Acquired immunodeficiency syndrome (AIDS)
[edit]Acquired immunodeficiency syndrome (AIDS) is a chronic condition caused by the infection of human immunodeficiency virus (HIV).[13] HIV is a sexually transmitted disease that can be spread by contact with infected blood. Additionally, it can also be spread from the mother to child during pregnancy, childbirth or breast-feeding.[13] [14] In the later stages, HIV can weaken a person's immune system by targeting CD4 cells (T cells), a type of white blood cell. CD4 cells play a major role in destroying pathogens and fighting infections. Hence, its loss of function causes vulnerability in the body and leads to serious complications.[14][15]
Highly active antiretroviral therapy (HAART)
[edit]
The highly active antiretroviral therapy (HAART) is the standard therapy for treatment of AIDS/HIV today. The treatment comprises a few classes of antiviral drugs which include non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), entry inhibitors and protease inhibitors (PI).[16] The HAART hinders the replication of HIV in the patient's body by blocking some stages in the replication cycle of HIV. NNRTIs and NRTIs can bind to HIV reverse transcriptase (HIV enzyme) and block the reverse transcription of HIV. As a result, the conversion of RNA to DNA is inhibited and HIV replication is terminated.[17][18] Entry inhibitors can bind to proteins on the surface of CD4 cells or HIV. This can prevent the outer coat of HIV from binding to the proteins on the surface of CD4 cells. The ability of HIV to penetrate CD4 cells is hindered, hence it is not able to infect healthy CD4 cells.[19] Protease inhibitors (PI) binds to protease (HIV enzyme) and impedes the cleavage of protein precursors, a process in the synthesis of HIV. As a result, the HIV formed will be defective and they are unable to bind to and infect CD4 cells.[20] The combined action of the drugs can lower the level of the HIV (viral load) in the body to ‘undetectable’ levels. Life long treatment is necessary to prolong the life expectancy of people infected with HIV.[16]
Some adverse effects of the treatment include dyslipidaemia, neutropenia and osteoporosis.[21]
Genital herpes
[edit]Genital herpes is an infection caused by the herpes simplex virus in a person’s body. There are 2 types of herpes simplex virus: herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2). HSV-1 is typically transferred through oral-to-oral contact and causes oral herpes. It can also be spread through genital-to-genital contact, causing genital herpes. HSV-2 is exclusively transferred through sexual activity. During initial infection, most people are asymptomatic. Symptoms of genital herpes include small red bumps, tiny white blisters and scabs at the site of infection. Genital herpes is one of the most common STDs in the world.[22]

Acyclovir
[edit]Acyclovir, also termed as Aciclovir, is an antiviral drug taken orally for the treatment of genital herpes.[20] Acyclovir is converted to acyclovir triphosphate by the host cell enzymes. Acyclovir triphosphate binds to the herpes simplex virus DNA polymerase and prevents viral DNA synthesis. Subsequently, deoxyguanosine triphosphate, a nucleotide precursor in herpes simplex virus for DNA synthesis, will not be able to bind to the viral DNA polymerase anymore. The viral replication of herpes simplex virus is impeded.[23] Acyclovir can decrease the pain and speed up the healing of the blisters at the site of infection.[20]
Common side effects of the drug include nausea, headache, vomiting and diarrhea.[24] It is recommended that people with kidney, bowel and heart problems should avoid taking Acyclovir as they are at risk of developing more severe side effects, including gastrointestinal bleeding and tachycardia.[25]
STDs caused by bacterial infection
[edit]The most common STDs caused by bacteria are chlamydia, chancroid, and gonorrhea. All of them are treatable by antibiotics.[26]
Chlamydia
[edit]Chlamydia is the most commonly reported STD in the US and it infects both men and women, with high prevalence among sexually active adolescents.[27] It is caused by the gram-negative bacterium Chlamydia trachomatis and can be spread during unprotected sex. It could be transmitted from an infected mother to the baby during labor.[28] Most people infected with chlamydia show no symptoms. However, women with symptoms may experience a burning sensation during urination and encounter an unusual vaginal discharge. Men with Chlamydia may encounter a discharge from their penis and feel pain in the testicles.[29]
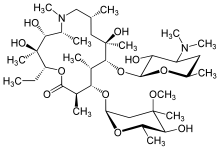

Azithromycin or Doxycycline
[edit]Chlamydia can be successfully cured with antibiotics. More than 95% of people can be cured if they take their antibiotics correctly.[30] Azithromycin and doxycycline are the two most commonly prescribed antibiotics. Both antibiotics are bacteriostatic as they stop the reproduction and growth of bacteria rather than killing it. Azithromycin binds to the 50S subunit of the bacterial ribosome, while doxycycline binds to the 30S ribosomal subunit. Both of them interfere protein synthesis in bacteria by inhibiting the translation of mRNA.[31]
Side effects of Azithromycin include nausea and abdominal pain while that of doxycycline includes diarrhea, esophageal ulcer and headache. Azithromycin is given as two or four tablets at once, while doxycycline are given two capsules a day for a week.[32] For patients who are pregnant, breastfeeding, or allergic, other probiotics such as Amoxicillin or Erythromycin may be prescribed.[33] If left untreated, chlamydia may spread to the uterus and fallopian tubes, causing pelvic inflammatory disease (PID) in females and epididymo-orchitis in males. PID causes permanent damage in women’s reproductive system and lead to infertility and ectopic pregnancy. Men rarely have health problems linked to chlamydia.[28]
Chancroid
[edit]Chancroid is a sexually transmitted disease caused by infection with Haemophilus ducreyi, a gram negative bacteria.[34] It is spread through sexual transmission and infects both male and female. Despite being rare developing countries such as in Europe and the United States, it is a major STD in many developing countries, with roughly seven million cases observed worldwide.[35]
Symptoms of chancroid include painful, red-colored bumps in the genital region which could eventually become open sores. Chancroid sores are more painful in men but less noticeable in women.[36]
Ciprofloxacin
[edit]Haemophilus ducreyi can be cured completely by antibiotics like ciprofloxacin.[37]Ciprofloxacin is a fluoroquinolone, which is an antibiotic that destroys the bacteria by interfering its cell division.[38] Ciprofloxacin inhibits DNA gyrase, an enzyme required for bacterial DNA replication. Additionally, ciprofloxacin has a half‐life of four hours. 40% to 50% of the oral dose is excreted in the urine as an unmetabolized drug. Its common side effects include nausea and diarrhea.[39] Severe adverse effects include increased risk in tendinitis, tendon rupture and difficulty in breathing.[40] Furthermore, it may even cause nerve damage or worsen muscle weakness in myasthenia gravis patients.[41] Ciprofloxacin may lead to problems with tissues, bones and joints in children. Therefore, they should not be prescribed to children younger than 18 unless they have certain serious infections that cannot be treated with other antibiotics.[39][42]
Gonorrhea
[edit]
Gonorrhea is the second most prevalent sexually transmitted disease caused by bacteria.[43] It is caused by the bacterium Neisseria gonorrhoeae.[44] It is transmitted during sexual contact and infects both males and females, affecting the urethra, rectum and throat. Gonorrhea can also be transmitted when babies' eyes are in contact with the birth canal of an infected mother.[43][45]
Gonorrhea is usually asymptomatic. However, it can affect the genital tract and cause painful urination or pelvic pain. Additionally, it also affects other parts of the body like rectum, throat, and joints.[46]
Untreated gonorrhea leads to major complications, for example:
- Infertility in women[46]
- Infertility in men[47]
- Increased risk of HIV/AIDS[48]
- Complications in babies[49]

Ceftriaxone and Azithromycin
[edit]Due to emerging strains of Neisseria gonorrhoeae drug-resistance, the Centers for Disease Control and Prevention recommends gonorrhea to be treated with both the injected antibiotic ceftriaxone, and orally taken azithromycin.[50]
Ceftriaxone destroys Neisseria gonorrhoeae by inhibiting the mucopeptide synthesis in bacterial cell wall.[51] The β-lactam moiety of ceftriaxone binds to enzymes involved in Neisseria gonorrhoeae cell-wall synthesis and cell division, including carboxypeptidases, endopeptidases, and transpeptidases.[52]
For the past 70–80 years, Neisseria gonorrhoeae has developed resistance to almost all antibiotics introduced, including penicillin and ciprofloxacin. Gonorrhea may become untreatable in certain circumstances in the near future due to the lack of treatment options.[53]
References
[edit]- ^ a b Tsevat, Danielle G.; Wiesenfeld, Harold C.; Parks, Caitlin; Peipert, Jeffrey F. (2017-01-01). "Sexually transmitted diseases and infertility". American Journal of Obstetrics & Gynecology. 216 (1): 1–9. doi:10.1016/j.ajog.2016.08.008. ISSN 0002-9378. PMC 5193130. PMID 28007229.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Nijmeijer, Bernadien M.; Koopsen, Jelle; Schinkel, Janke; Prins, Maria; Geijtenbeek, Teunis BH (2019). "Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men". Journal of the International AIDS Society. 22 (S6): e25348. doi:10.1002/jia2.25348. ISSN 1758-2652. PMC 6715947. PMID 31468692.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b "Sexually Transmitted Diseases". medlineplus.gov. 3 March 2021. Retrieved 2021-03-29.
{{cite web}}: CS1 maint: url-status (link) - ^ a b "Treatments for Sexually Transmitted Diseases (STDs)". WebMD. Retrieved 2021-03-26.
- ^ Croxford, Sara; Kitching, Aileen; Desai, Sarika; Kall, Meaghan; Edelstein, Michael; Skingsley, Andrew; Burns, Fiona; Copas, Andrew; Brown, Alison E.; Sullivan, Ann K.; Delpech, Valerie (2017-01-01). "Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort". The Lancet Public Health. 2 (1): e35–e46. doi:10.1016/S2468-2667(16)30020-2. ISSN 2468-2667. PMID 29249478.
- ^ Nabovati, Ehsan; TaherZadeh, Zhila; Eslami, Saeid; Abu-Hanna, Ameen; Abbasi, Reza (2021-01-14). "Antibiotic prescribing in inpatient and outpatient settings in Iran: a systematic review and meta-analysis study". Antimicrobial Resistance & Infection Control. 10 (1): 15. doi:10.1186/s13756-021-00887-x. ISSN 2047-2994. PMC 7809737. PMID 33446279.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b Gabster, Amanda; Pascale, Juan Miguel; Cislaghi, Beniamino; Francis, Suzanna C.; Weiss, Helen A.; Martinez, Alexander; Ortiz, Alma; Herrera, Mellissa; Herrera, Genarino; Gantes, Cesar; Quiel, Yaremis (December 2019). "High Prevalence of Sexually Transmitted Infections, and High-Risk Sexual Behaviors Among Indigenous Adolescents of the Comarca Ngäbe-Buglé, Panama". Sexually Transmitted Diseases. 46 (12): 780–787. doi:10.1097/OLQ.0000000000001070. ISSN 0148-5717.
- ^ a b CDC (2021-02-10). "Hepatitis B | CDC". Centers for Disease Control and Prevention. Retrieved 2021-03-26.
- ^ a b c Cai, Shaohang; Cao, Jiawei; Yu, Tao; Xia, Muye; Peng, Jie (2017-06-02). "Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine". Medicine. 96 (22). doi:10.1097/MD.0000000000007021. ISSN 0025-7974. PMC 5459719. PMID 28562554.
- ^ a b Tang, Lydia S. Y.; Covert, Emily; Wilson, Eleanor; Kottilil, Shyam (2018-05-01). "Chronic Hepatitis B Infection: A Review". JAMA. 319 (17): 1802–1813. doi:10.1001/jama.2018.3795. ISSN 0098-7484.
- ^ Choi, Won-Mook; Choi, Jonggi; Lim, Young-Suk (2021-02-01). "Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection: A Systematic Review and Meta-analysis". Clinical Gastroenterology and Hepatology. 19 (2): 246–258.e9. doi:10.1016/j.cgh.2020.05.008. ISSN 1542-3565. PMID 32407970.
- ^ "Entecavir Monograph for Professionals". Drugs.com. 23 October 2017. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Cao, Wei; Mehraj, Vikram; Kaufmann, Daniel E.; Li, Taisheng; Routy, Jean-Pierre (2016). "Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era". Journal of the International AIDS Society. 19 (1): 20697. doi:10.7448/IAS.19.1.20697. ISSN 1758-2652. PMC 4779330. PMID 26945343.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Cao, Wei; Mehraj, Vikram; Kaufmann, Daniel E.; Li, Taisheng; Routy, Jean-Pierre (2016). "Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era". Journal of the International AIDS Society. 19 (1): 20697. doi:10.7448/IAS.19.1.20697. ISSN 1758-2652. PMC 4779330. PMID 26945343.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Kaplan, Jonathan (23 June 2019). "How CD4 Counts Help Treat HIV". WebMD. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Chan, Kenny CW (2020). "11. ANTIRETROVIRAL THERAPY". www.aids.gov.hk. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ "Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) | ClinicalInfo". clinicalinfo.hiv.gov. 2021. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ "Nucleoside Reverse Transcriptase Inhibitors ~ ViralZone". viralzone.expasy.org. 2020. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ Sepúlveda‐Crespo, Daniel; Ceña‐Díez, Rafael; Jiménez, José Luis; Muñoz‐Fernández, Ma Ángeles (2017). "Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections". Medicinal Research Reviews. 37 (1): 149–179. doi:10.1002/med.21405. ISSN 1098-1128.
- ^ a b c "Acyclovir: MedlinePlus Drug Information". medlineplus.gov. 2020. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ Silva, B. F.; Peixoto, Gml; da Luz, S. R.; de Moraes, Smf; Peres, S. B. (22 April 2019). "Adverse effects of chronic treatment with the Main subclasses of highly active antiretroviral therapy: a systematic review". HIV medicine. 20 (7): 429–438. doi:10.1111/hiv.12733. ISSN 1468-1293. PMID 31006976.
- ^ "Herpes simplex virus". www.who.int. 1 May 2020. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ Sepúlveda‐Crespo, Daniel; Ceña‐Díez, Rafael; Jiménez, José Luis; Muñoz‐Fernández, Ma Ángeles (2017). "Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections". Medicinal Research Reviews. 37 (1): 149–179. doi:10.1002/med.21405. ISSN 1098-1128.
- ^ Cunha, John (13 January 2021). "Acyclovir (Zovirax): Side Effects, Dosages, Treatment, Interactions, Warnings". RxList. Retrieved 2021-03-26.
{{cite web}}: CS1 maint: url-status (link) - ^ Chang, Hua-Ching; Sung, Chih-Wei; Lin, Ming-Hsiu (4 September 2018). "The efficacy of oral acyclovir during early course of pityriasis rosea: a systematic review and meta-analysis". Journal of Dermatological Treatment. 30 (3): 288–293. doi:10.1080/09546634.2018.1508820. ISSN 0954-6634. PMID 30109959.
- ^ Davey, DL Joseph; Shull, HI; Billings, JD; Wang, D; Adachi, K; Klausner, JD (July 2016). "Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015". Sexually transmitted diseases. 43 (7): 450–458. doi:10.1097/OLQ.0000000000000460. ISSN 0148-5717. PMC 5889114. PMID 27322048.
- ^ "Chlamydia - 2017 Sexually Transmitted Diseases Surveillance". www.cdc.gov. 2019-01-11. Retrieved 2021-03-31.
- ^ a b "Chlamydia trachomatis | Red Book® 2018 | Red Book Online | AAP Point-of-Care-Solutions". redbook.solutions.aap.org. Retrieved 2021-04-14.
- ^ "STD Facts - Chlamydia". www.cdc.gov. 2020-08-05. Retrieved 2021-04-14.
- ^ "Chlamydia - Treatment". nhs.uk. 2017-10-20. Retrieved 2021-03-29.
- ^ Sandman, Zachary; Iqbal, Omar A. (2021), "Azithromycin", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32491698, retrieved 2021-04-14
- ^ "Azithromycin", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31643753, retrieved 2021-04-14
- ^ "(PDF) The Efficacy of Antibiotics in the Treatment of Chlamydia Trachomatis Infections during Pregnancy. A Systematic Review and a Meta-analysis". ResearchGate. Retrieved 2021-04-14.
- ^ "Sexually Transmitted Diseases Treatment Guidelines, 2015". www.cdc.gov. Retrieved 2021-04-14.
- ^ "Chancroid Epidemiology". News-Medical.net. 2017-10-09. Retrieved 2021-03-29.
- ^ Irizarry, Lisandro; Velasquez, James; Wray, Anton A. (2021), "Chancroid", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30020703, retrieved 2021-04-14
- ^ Thai, Tony; Salisbury, Blake H.; Zito, Patrick M. (2021), "Ciprofloxacin", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30571075, retrieved 2021-04-14
- ^ Fasugba, Oyebola; Gardner, Anne; Mitchell, Brett G.; Mnatzaganian, George (2015-11-25). "Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies". BMC Infectious Diseases. 15. doi:10.1186/s12879-015-1282-4. ISSN 1471-2334. PMC 4660780. PMID 26607324.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b "Ciprofloxacin: MedlinePlus Drug Information". medlineplus.gov. Retrieved 2021-03-29.
- ^ Lautenschlager, Stephan; Kemp, Michael; Christensen, Jens Jørgen; Mayans, Marti Vall; Moi, Harald (2017-03-01). "2017 European guideline for the management of chancroid". International Journal of STD & AIDS. 28 (4): 324–329. doi:10.1177/0956462416687913. ISSN 0956-4624.
- ^ "Side Effects of Cipro (Ciprofloxacin), Warnings, Uses". RxList. Retrieved 2021-04-14.
- ^ Thai, Tony; Salisbury, Blake H.; Zito, Patrick M. (2021), "Ciprofloxacin", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30571075, retrieved 2021-04-14
- ^ a b Sanchez, Juan David (2018-12-12). "PAHO/WHO, Gonorrhea". Pan American Health Organization / World Health Organization.
{{cite web}}: CS1 maint: url-status (link) - ^ Pillay, Jennifer; Moore, Ainsley; Rahman, Prinon; Lewin, Gabriel; Reynolds, Donna; Riva, John; Thériault, Guyléne; Thombs, Brett; Wilson, Brenda; Robinson, Joan; Ramdyal, Amanda (2018-12-26). "Screening for chlamydia and/or gonorrhea in primary health care: protocol for systematic review". Systematic Reviews. 7 (1): 248. doi:10.1186/s13643-018-0904-5. ISSN 2046-4053. PMC 6307186. PMID 30587234.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ "Gonococcal Infections | Red Book® 2018 | Red Book Online | AAP Point-of-Care-Solutions". redbook.solutions.aap.org. Retrieved 2021-04-14.
- ^ a b Unemo, Magnus; Seifert, H. Steven; Hook, Edward W.; Hawkes, Sarah; Ndowa, Francis; Dillon, Jo-Anne R. (2019-11-21). "Gonorrhoea". Nature Reviews Disease Primers. 5 (1): 1–23. doi:10.1038/s41572-019-0128-6. ISSN 2056-676X.
- ^ TSEVAT, Danielle G.; WIESENFELD, Harold C.; Parks, Caitlin; PEIPERT, Jeffrey F. (2017-1). "Sexually Transmitted Diseases and Infertility". American journal of obstetrics and gynecology. 216 (1): 1–9. doi:10.1016/j.ajog.2016.08.008. ISSN 0002-9378. PMC 5193130. PMID 28007229.
{{cite journal}}: Check date values in:|date=(help) - ^ Pines, Heather A.; Wertheim, Joel O.; Liu, Lin; Garfein, Richard S.; Little, Susan J.; Karris, Maile Y. (2016-11-28). "Concurrency and HIV transmission network characteristics among MSM with recent HIV infection". AIDS (London, England). 30 (18): 2875–2883. doi:10.1097/QAD.0000000000001256. ISSN 1473-5571. PMC 5279958. PMID 27662550.
- ^ Pillay, Jennifer; Moore, Ainsley; Rahman, Prinon; Lewin, Gabriel; Reynolds, Donna; Riva, John; Thériault, Guyléne; Thombs, Brett; Wilson, Brenda; Robinson, Joan; Ramdyal, Amanda (2018-12-26). "Screening for chlamydia and/or gonorrhea in primary health care: protocol for systematic review". Systematic Reviews. 7 (1): 248. doi:10.1186/s13643-018-0904-5. ISSN 2046-4053. PMC 6307186. PMID 30587234.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ "CDC - Gonorrhea Treatment". www.cdc.gov. 2020-12-16. Retrieved 2021-04-14.
- ^ Costa-Lourenço, Ana Paula Ramalho da; Barros dos Santos, Késia Thaís; Moreira, Beatriz Meurer; Fracalanzza, Sergio Eduardo Longo; Bonelli, Raquel Regina (2017-07-12). "Antimicrobial resistance in Neisseria gonorrhoeae: history, molecular mechanisms and epidemiological aspects of an emerging global threat". Brazilian Journal of Microbiology. 48 (4): 617–628. doi:10.1016/j.bjm.2017.06.001. ISSN 1517-8382. PMC 5628311. PMID 28754299.
- ^ Pérez Medina, Krizia M.; Dillard, Joseph P. (2018-07-21). "Antibiotic Targets in Gonococcal Cell Wall Metabolism". Antibiotics. 7 (3). doi:10.3390/antibiotics7030064. ISSN 2079-6382. PMC 6164560. PMID 30037076.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Basic Information about ARG - STD information from CDC". www.cdc.gov. 2020-12-15. Retrieved 2021-04-14.
