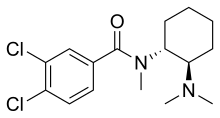
U-47700
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H22Cl2N2O |
| Molar mass | 329.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
U-47700, also known as U4, pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s[1] which has around 7.5 times the potency of morphine in animal models.[2][3][4]

U-47700 is a structural isomer of the earlier opioid AH-7921[6] and the result of a great deal of work elucidating the quantitative structure–activity relationship of the scaffold. Upjohn looked for the key moieties which gave the greatest activity[7] and posted over a dozen patents on related compounds, each optimizing one moiety[8][9][10][11][12][13][14][15] until they discovered that U-47700 was the most active.[16]
U-47700 became the lead compound of selective kappa-opioid receptor ligands such as U-50488, U-51754 (containing a pyrrolidine rather than a dimethylamine substituent) and U-69,593, which share very similar structures.[17][18] Although not used medically, the selective kappa ligands are used in research.[19][20]
Pharmacology
[edit]U-47700 is an agonist of the μ-opioid receptor (Ki 11.1 ± 0.4 nM) and possesses significantly lower affinity for the κ-opioid receptor (Ki = 287 ± 24 nM) and δ-opioid receptor (Ki = 1220 ± 82 nM). U-47700 is approximately 10-fold more potent than morphine in rats, although the binding of U-47700 is 2–4 times weaker than morphine at all three opioid receptors.[21]
The metabolism of U-47700 in humans involves mono- and didesmethylation followed by hydroxylation.[22] The desmethyl metabolites of U-47700 have negligible affinity for the opioid receptors and are not thought to contribute to the activity of U-47700.[23][24]
Side effects
[edit]U-47700 has never been studied in humans, but it would be expected to produce effects similar to those of other potent opioid agonists, including strong analgesia, sedation, euphoria, constipation, itching and respiratory depression which could be harmful or fatal.[25][26][27][28][29][30][31][32] Tachycardia was another side effect encountered with U-47700 use.[32] Tolerance and dependence would be expected to develop.[33]
Deaths
[edit]Combined consumption of U-47700 with fentanyl and flubromazepam caused one fatality each in Belgium and Germany, respectively.[34][35][36] One death was reported in Ireland [37] and another one in Italy.[38] 17 opioid overdoses and several deaths in the United States had initially been associated with U-47700 in April 2016.[39] As of September 2016 at least 15 fatalities were confirmed. By December 2017, at least 46 fatalities had been associated with the use of U-47700.[40][41][42][43][44][45][46]
U-47700 was found in combination with fentanyl during the autopsy of the American artist Prince in 2016.[47]
Detection in biological fluids
[edit]U-47700 may be measured in serum, plasma, blood or urine to monitor for abuse, confirm a diagnosis of poisoning, or assist in a medicolegal death investigation. Serum or blood U-47700 concentrations are expected to be in a range of 10–250 μg/L in intoxicated patients and 100–1,500 μg/L in deceased victims of acute overdosage. The detection usually involves analysis by liquid chromatography-mass spectrometry.[48]
Society and culture
[edit]Common street names for U-47700 include pinky, pink, and U4.[49]
Legal status
[edit]Following its sale as a designer drug, U-47700 was made illegal in Sweden on January 26, 2016.[50]
U-47700 was emergency scheduled in Ohio on May 3, 2016, by executive order of Governor John Kasich.[51]
U-47700 was emergency scheduled in Florida on September 27, 2016, by an emergency rule of Florida Attorney General Pam Bondi.[52]
Responding to a perceived threat to public health and safety, the U.S. Drug Enforcement Administration has placed U-47700 into Schedule I of the Controlled Substances Act, effective November 14, 2016.[53] In April 2018, U-47700 was placed into Schedule I indefinitely.[54]
U-47700 was placed into Schedule 1 of South Dakota's Controlled Substance Schedule. It was signed by Governor Daugaard on February 9, 2017.[55]
U-47700 was made a Class A, Schedule 1 drug under the Misuse of Drugs Act in the UK in 2017.[56]
In popular culture
[edit]U-47700 is the title of a 2021 Dutch sci-fi short film by director Erasmo de la Parra.[57] In the film, the characters are addicted to a new drug containing the substance U-47700.
See also
[edit]References
[edit]- ^ Szmuszkovicz J (4 July 1978). "Patent US4098904 - Analgesic N-(2-aminocycloaliphatic)benzamides".
- ^ Cheney BV, Szmuszkovicz J, Lahti RA, Zichi DA (December 1985). "Factors affecting binding of trans-N-[2-(methylamino)cyclohexyl]benzamides at the primary morphine receptor". Journal of Medicinal Chemistry. 28 (12): 1853–1864. doi:10.1021/jm00150a017. PMID 2999404.
- ^ Harper NJ, Veitch GB, Wibberley DG (November 1974). "1-(3,4-Dichlorobenzamidomethyl)cyclohexyldimethylamine and related compounds as potential analgesics". Journal of Medicinal Chemistry. 17 (11): 1188–1193. doi:10.1021/jm00257a012. PMID 4416926.
- ^ Szmuszkovicz J, Von Voigtlander PF (October 1982). "Benzeneacetamide amines: structurally novel non-m mu opioids". Journal of Medicinal Chemistry. 25 (10): 1125–1126. doi:10.1021/jm00352a005. PMID 6128415.
- ^ Alzghari SK, Fleming SW, Rambaran KA, Long JE, Burkhart S, An J, Furmaga J (October 2017). "U-47700: An Emerging Threat". Cureus. 9 (10): e1791. doi:10.7759/cureus.1791. PMC 5741271. PMID 29282436.
- ^ Brittain RT, Kellett DN, Neat ML, Stables R (September 1973). "Proceedings: Anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides". British Journal of Pharmacology. 49 (1): 158P – 159P. doi:10.1111/j.1476-5381.1973.tb08279.x. PMC 1776456. PMID 4207044.
- ^ Michalson ET, Szmuszkovicz J (1989). "Medicinal agents incorporating the 1,2-diamine functionality". Progress in Drug Research. Vol. 33. Birkhäuser Basel. pp. 135–149. doi:10.1007/978-3-0348-9146-2_6. ISBN 9783034891462. PMID 2687936.
{{cite book}}:|journal=ignored (help) - ^ Mullins DD (28 June 1966). "Patent US US3258489 - N-(1-aminocyclohexylmethyl)anilines and N-(1-nitrocyclohexylmethyl)anilines".
- ^ Harper NJ, Veitch GB (17 August 1976). "Patent US3975443 - 1-(3,4-dichlorobenzamidomethyl)-cyclohexyldimethylamine".
- ^ Szmuszkovicz J (3 March 1970). "Patent US3499033 - Ethers of α-phenyl-2-aminocycloalkanemethanols".
- ^ Szmuszkovicz J (5 May 1970). "Patent US3510492 - 2-anilino and 2-anilinomethyl cycloalkylamines".
- ^ Rynbrandt RH, Skaletzky LL (7 March 1972). "Patent US3647804 - Cycloalkanecarboxamides".
- ^ Roll W (23 July 1974). "Patent US3825595 - N-cyclopentyl-N-2-hydroxyalkyl-ring-substituted benzamides".
- ^ Harper NJ, Veitch GB (20 September 1977). "Patent US4049663 - Ethylene diamine derivatives".
- ^ Collins RJ, Kaplan LJ, Ludens JH, Von Voigtlander PF (9 April 1982). "Patent US4463013 - Oxygen substituted amino-cyclohexyl-benzeneacetamides and -benzamides as water diuretic drugs".
- ^ Casy AF, Parfitt RT (1986). "Miscellaneous Groups of Analgesics". Opioid Analgesics. Springer US. pp. 385–403. doi:10.1007/978-1-4899-0585-7_11. ISBN 9781489905857.
- ^ Szmuszkovicz J, Zhao S, Totleben MJ, Mizsak SA, Freeman JP (2000). "Phenanthridone Analogs of the Opiate Agonist U-47,700 in the trans-1,2-Diaminocyclohexane Benzamide Series". Heterocycles. 52 (1): 325–332. doi:10.3987/com-99-s27.
- ^ Loew G, Lawson J, Toll L, Frenking G, Berzetei-Gurske I, Polgar W (1988). "Structure activity studies of two classes of beta-amino-amides: the search for kappa-selective opioids" (PDF). NIDA Research Monograph. 90: 144–151. PMID 2855852.
- ^ Szmuszkovicz J (1999). "U-50,488 and the к receptor: A personalized account covering the period 1973 to 1990". Progress in Drug Research. Vol. 52. Birkhäuser Basel. pp. 167–195. doi:10.1007/978-3-0348-8730-4_4. ISBN 9783034887304. PMID 10396128.
{{cite book}}:|journal=ignored (help) - ^ Tsibulnikov SY, Maslov LN, Mukhomedzyanov AV, Krylatov AV, Tsibulnikova MR, Lishmanov YB (October 2015). "Prospects of Using of κ-Opioid Receptor Agonists U-50,488 and ICI 199,441 for Improving Heart Resistance to Ischemia/Reperfusion". Bulletin of Experimental Biology and Medicine. 159 (6): 718–721. doi:10.1007/s10517-015-3057-8. PMID 26519268. S2CID 1046853.
- ^ Truver MT, Smith CR, Garibay N, Kopajtic TA, Swortwood MJ, Baumann MH (October 2020). "Pharmacodynamics and pharmacokinetics of the novel synthetic opioid, U-47700, in male rats". Neuropharmacology. 177: 108195. doi:10.1016/j.neuropharm.2020.108195. PMC 7554234. PMID 32533977. S2CID 219559700.
- ^ Krotulski AJ, Mohr AL, Papsun DM, Logan BK (January 2018). "Metabolism of novel opioid agonists U-47700 and U-49900 using human liver microsomes with confirmation in authentic urine specimens from drug users". Drug Testing and Analysis. 10 (1): 127–136. doi:10.1002/dta.2228. PMID 28608586.
- ^ Nordmeier F, Cannaert A, Stove CP, Schmidt PH, Meyer MR, Schaefer N (April 2022). "Are the N-demethylated metabolites of U-47700 more active than their parent compound? In vitro μ-opioid receptor activation of N-desmethyl-U-47700 and N,N-bisdesmethyl-U-47700". Drug Testing and Analysis. 14 (4): 713–717. doi:10.1002/dta.3182. hdl:1854/LU-8751266. PMID 34669261.
- ^ Truver MT, Smith CR, Garibay N, Kopajtic TA, Swortwood MJ, Baumann MH (October 2020). "Pharmacodynamics and pharmacokinetics of the novel synthetic opioid, U-47700, in male rats". Neuropharmacology. 177: 108195. doi:10.1016/j.neuropharm.2020.108195. PMC 7554234. PMID 32533977. S2CID 219559700.
- ^ Davis TL (9 March 2016). "New Synthetic Opioid Surfaces in North Texas". NBCDFW.
- ^ Jones MJ, Hernandez BS, Janis GC, Stellpflug SJ (January 2017). "A case of U-47700 overdose with laboratory confirmation and metabolite identification". Clinical Toxicology. 55 (1): 55–59. doi:10.1080/15563650.2016.1209767. PMID 27549165. S2CID 27920117.
- ^ Domanski K, Kleinschmidt KC, Schulte JM, Fleming S, Frazee C, Menendez A, Tavakoli K (January 2017). "Two cases of intoxication with new synthetic opioid, U-47700". Clinical Toxicology. 55 (1): 46–50. doi:10.1080/15563650.2016.1209763. PMID 27432224. S2CID 46228909.
- ^ Ruan X, Chiravuri S, Kaye AD (September 2016). "Comparing fatal cases involving U-47700". Forensic Science, Medicine, and Pathology. 12 (3): 369–371. doi:10.1007/s12024-016-9795-8. PMID 27421264. S2CID 24201474.
- ^ Elliott SP, Brandt SD, Smith C (August 2016). "The first reported fatality associated with the synthetic opioid 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700) and implications for forensic analysis" (PDF). Drug Testing and Analysis. 8 (8): 875–879. doi:10.1002/dta.1984. PMID 27232154. S2CID 205762837.
- ^ Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR (January 2017). "Fentanyl and a Novel Synthetic Opioid U-47700 Masquerading as Street "Norco" in Central California: A Case Report". Annals of Emergency Medicine. 69 (1): 87–90. doi:10.1016/j.annemergmed.2016.06.014. PMID 27473610.
- ^ Helander A, Bäckberg M (January 2017). "New Psychoactive Substances (NPS) - the Hydra monster of recreational drugs". Clinical Toxicology. 55 (1): 1–3. doi:10.1080/15563650.2016.1217003. PMID 27549399. S2CID 35645218.
- ^ a b Rambaran KA, Fleming SW, An J, Burkhart S, Furmaga J, Kleinschmidt KC, et al. (October 2017). "U-47700: A Clinical Review of the Literature". The Journal of Emergency Medicine. 53 (4): 509–519. doi:10.1016/j.jemermed.2017.05.034. PMID 28911989.
- ^ Kimergård A, Breindahl T, Hindersson P, Deluca P (October 2016). "Tampering of opioid analgesics: a serious challenge for public health?". Addiction. 111 (10): 1701–1702. doi:10.1111/add.13436. PMID 27273814.
- ^ Coopman V, Blanckaert P, Van Parys G, Van Calenbergh S, Cordonnier J (September 2016). "A case of acute intoxication due to combined use of fentanyl and 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700)". Forensic Science International. 266: 68–72. doi:10.1016/j.forsciint.2016.05.001. hdl:1854/LU-8509152. PMID 27235591.
- ^ "Twee doden in België door overdosis met fentanylpleisters" (in Dutch). Deredactie. 29 January 2016.
- ^ Koch K, Auwärter V, Hermanns-Clausen M, Wilde M, Neukamm MA (April 2018). "Mixed intoxication by the synthetic opioid U-47700 and the benzodiazepine flubromazepam with lethal outcome: Pharmacokinetic data". Drug Testing and Analysis. 10 (8): 1336–1341. doi:10.1002/dta.2391. PMID 29637722.
- ^ "Young people playing Russian roulette with drugs, warns coroner". The Irish Times.
- ^ "Torino, ucciso dalla droga comprata online: la prima vittima italiana dell'U47700". LaStampa.it (in Italian). Retrieved 2017-10-20.
- ^ Zalkind S (11 April 2016). "Synthetic opiate makers stay step ahead of US drug laws as overdose cases rise". The Guardian. ISSN 0261-3077.
- ^ Draper B (6 June 2016). "New synthetic drug U-47700 has states rushing to stop spread". Associated Press.
- ^ Schneir A, Metushi IG, Sloane C, Benaron DJ, Fitzgerald RL (January 2017). "Near death from a novel synthetic opioid labeled U-47700: emergence of a new opioid class". Clinical Toxicology. 55 (1): 51–54. doi:10.1080/15563650.2016.1209764. PMID 27448790. S2CID 25206275.
- ^ Mohr AL, Friscia M, Papsun D, Kacinko SL, Buzby D, Logan BK (November 2016). "Analysis of Novel Synthetic Opioids U-47700, U-50488 and Furanyl Fentanyl by LC-MS/MS in Postmortem Casework". Journal of Analytical Toxicology. 40 (9): 709–717. doi:10.1093/jat/bkw086. PMID 27590036.
- ^ "19 Recent Deaths Associated With Synthetic Opioids; State Officials Urge Awareness". NC Department of Health and Human Services. 24 March 2016.
- ^ Drug Enforcement Administration (DEA) (7 September 2016). "Proposed Rule: Schedules of Controlled Substances: Temporary Placement of U-47700 Into Schedule I". Federal Register.
- ^ Krotulski AJ, Mohr AL, Papsun DM, Logan BK (January 2018). "Metabolism of novel opioid agonists U-47700 and U-49900 using human liver microsomes with confirmation in authentic urine specimens from drug users". Drug Testing and Analysis. 10 (1): 127–136. doi:10.1002/dta.2228. PMID 28608586.
- ^ Moody MT, Diaz S, Shah P, Papsun D, Logan BK (September 2018). "Analysis of fentanyl analogs and novel synthetic opioids in blood, serum/plasma, and urine in forensic casework". Drug Testing and Analysis. 10 (9): 1358–1367. doi:10.1002/dta.2393. PMID 29633785. S2CID 4758125.
- ^ "Furanyl Fentanyl Joins U-47,700 As The Second Illicit Opioid Banned By DEA In November". Forbes.
- ^ Baselt, R. (2017) Disposition of Toxic Drugs and Chemicals in Man, 11th edition, Biomedical Publications, Foster City, CA, pp. 2208.
- ^ "Pinky". pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Retrieved 16 July 2020.
- ^ "31 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. November 2015.
- ^ Kasich JR (May 3, 2016). "Executive Order 2016-01K" (PDF). Governor of Ohio.
- ^ "News Release - Attorney General Bondi Outlaws Deadly Synthetic Drug". www.myfloridalegal.com. Retrieved 2016-09-27.
- ^ DEA Public Affairs (November 11, 2016). "46 confirmed deaths linked to dangerous opioid in '15 and '16 spark emergency action". DEA. Archived from the original on June 4, 2018. Retrieved November 11, 2016.
- ^ "Schedules of Controlled Substances: Placement of Butyryl Fentanyl and U-47700 Into Schedule I". Federal Register. 20 April 2018.
- ^ South Dakota Legislature. "House Bill No. 1041" (PDF). Governor Dauggard of South Dakota.
- ^ "EXPLANATORY MEMORANDUM TO THE MISUSE OF DRUGS ACT 1971 (AMENDMENT) ORDER 2017" (PDF).
- ^ Filmaffinity U-47700 (S) (2021), retrieved 2022-05-16
