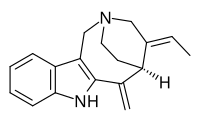
Apparicine
 | |
| Names | |
|---|---|
| IUPAC name
(19E)-2,7,16,17,19,20-Hexadehydro-3,7-seco-6-norcuran
| |
| Systematic IUPAC name
(2R,4E,5S)-4-Ethylidene-6-methylidene-1,3,4,5,6,7-hexahydro-2,5-ethanoazocino[4,3-b]indole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H20N2 | |
| Molar mass | 264.372 g·mol−1 |
| Density | 0.945875 |
| log P | 3.404 |
| Acidity (pKa) | 8.37 |
Refractive index (nD)
|
1.665 |
| 0.552121 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Apparicine is a monoterpenoid tricyclic indole alkaloid.[1] It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated.[2][3] It was the first member of the vallesamine group of indole alkaloids to be isolated and have its structure established,[3] which was first published in 1965.[4] It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.[5]
Biochemistry
[edit]
The alkaloid has been isolated from seven species of Aspidosperma.[6] It is the principal alkaloid found in the callus of Tabernaemontana elegans, and has also been identified in other Tabernaemontana species, including T. africana, T. divaricata, T. orientalis, and T. pachysiphon.[7][8] In studies of T. pachysiphon, it was found that alkaloid content including that of apparicine was greatest in young leaves and leaves receiving greater shade, and varied with leaf age, plant age, and provenance.[9]
Research on Aspidosperma pyricollum has led to the discovery that apparicine is biosynthesised from tryptophan by "loss of C-2 and retention of C-3".[10] The biosynthesis of apparicine requires alteration of the usual tryptamine side chain with loss of C-1.[1]
Structure determination
[edit]Its structure was established through the methods of chemical decomposition, and the nascent field of nuclear magnetic resonance (NMR) decoupling using the 1H isotope of hydrogen.[11] Ultraviolet–visible spectroscopy showed that apparicine has a similar UV absorption to uleine,[12] and their chromophores were found to be identical.[11]
NMR decoupling experiments revealed that apparicine lacks an N-methyl signal and has one methylenic carbon atom between the nitrogen atom and the indole rings, allowing researchers to distinguish it from uleine.[12] This was a notable early use of NMR decoupling to determine a chemical structure.[12] Its carbon skeleton was found to be related but different from that of uleine, and the structures of vallesamine and O-acetyl-vallesamine to be related to apparicine.[13]
Dehydrogenation of apparicine followed by oxidation with permanganate allowed location of the two piperidine ring carbon substituents.[14]
Applications
[edit]Apparicine may have several potential applications. In cell cultures, it has shown cytotoxicity against the experimental lymphocytic leukemia P388 cell line.[15] It exhibits strong activity against poliovirus type 3 (PV3),[15] and has moderate to strong activity against some human pathogens.[16] It is also active at opioid receptors[15] and has micromolar affinity for adenosine receptors.[17] Apparicine has local analgesic properties.[16] It inhibited xanthine oxidase as potently as allopurinol (IC50 = 0.65 μM).[18]
See also
[edit]Notes
[edit]- ^ a b Herbert 1983, p. 13.
- ^ Elia 2008, p. 594.
- ^ a b Joule 1983, p. 286.
- ^ Joule et al. 1965, p. 4773.
- ^ Gilbert 1968, p. 273.
- ^ Monteiro 1966, p. 39.
- ^ Verpoorte et al. 1989, p. 139.
- ^ Elia 2008, p. 593.
- ^ Elia 2008, p. 596.
- ^ Shamma 1970, p. 324.
- ^ a b Joule et al. 1980, p. 230.
- ^ a b c Joule 1983, p. 287.
- ^ Biemann 1966, p. 40.
- ^ Joule 1983, p. 288.
- ^ a b c Schmelzer 2008, p. 592.
- ^ a b Mairura & Schmelzer 2008, p. 590.
- ^ Ingkaninan et al. 1999, p. 1441.
- ^ Shi BB, Chen J, Bao MF, Zeng Y, Cai XH (October 2019). "Alkaloids isolated from Tabernaemontana bufalina display xanthine oxidase inhibitory activity". Phytochemistry. 166: 112060. doi:10.1016/j.phytochem.2019.112060. PMID 31302343. S2CID 196613130.
References
[edit]- Biemann, Klaus (1966). "Mass spectrometry of selected natural products". In Zechmeister, L. (ed.). Fortschritte der Chemie organischer Naturstoffe. Vol. 24. Springer-Verlag. pp. 2–98. doi:10.1007/978-3-7091-8143-0_1. ISBN 978-3-7091-8145-4. PMID 5958065.
- Elia, J. (2008). "Tabernaemontana pachysiphon Stapf". In Schmelzer, G. H.; Gurib-Fakim, A. (eds.). Medicinal Plants 1. Plant Resources of Tropical Africa. Vol. 11. PROTA Foundation; Backhuys Publishers; CTA. pp. 593–596. ISBN 978-90-5782-204-9.
- Gilbert, B. (1968). "The alkaloids of Aspidosperma, Ochrosia, Pleiocarpa, Melodinus, and related genera". In Manske, R. H. F. (ed.). The Alkaloids: Chemistry and Physiology. Vol. 11. Academic Press. pp. 205–306. doi:10.1016/S1876-0813(08)60121-9. ISBN 978-0-12-469511-5.
- Herbert, Richard B. (1983). "Structural and biosynthetic relationships". In Saxton, J. Edwin (ed.). Indoles: Part Four: The Monoterpenoid Indole Alkaloids. The Chemistry of Heterocyclic Compounds. Vol. 25. John Wiley & Sons. pp. 1–46. doi:10.1002/9780470186954.ch1. ISBN 0-471-89748-5.
- Ingkaninan, K.; Ijzerman, A. P.; Taesotikul, T.; Verpoorte, R. (1999). "Isolation of opioid-active compounds from Tabernaemontana pachysiphon leaves". Journal of Pharmacy and Pharmacology. 51 (12): 1441–1446. doi:10.1211/0022357991777092. PMID 10678501. S2CID 45544097.
- Joule, John A. (1983). "The uleine–ellipticine–vallesamine group". In Saxton, J. Edwin (ed.). Indoles: Part Four: The Monoterpenoid Indole Alkaloids. The Chemistry of Heterocyclic Compounds. Vol. 25. John Wiley & Sons. pp. 265–292. doi:10.1002/9780470186954.ch1. ISBN 0-471-89748-5.
- Joule, J. A.; Allen, M. S.; Bishop, D. I.; Harris, M.; et al. (1980). "Approaches to the synthesis of apparicine". In Phillipson, John David; Zenk, M. H. (eds.). Indole and Biogenetically Related Alkaloids. Annual Proceedings of the Phytochemical Society of Europe. Vol. 17. Academic Press. pp. 229–248. ISBN 0-12-554450-2.
- Joule, J. A.; Monteiro, H.; Durham, L. J.; Gilbert, B.; et al. (1965). "Alkaloid studies. Part XLVIII. The structure of apparicine, a novel Aspidosperma alkaloid". Journal of the Chemical Society (4): 4773–4780. doi:10.1039/JR9650004773. PMID 5891947.
- Mairura, F. S.; Schmelzer, G. H. (2008). "Tabernaemontana crassa Benth.". In Schmelzer, G. H.; Gurib-Fakim, A. (eds.). Medicinal Plants 1. Plant Resources of Tropical Africa. Vol. 11. PROTA Foundation; Backhuys Publishers; CTA. pp. 589–592. ISBN 978-90-5782-204-9.
- Monteiro, Hugo Jorge (1966). Studies on some indole alkaloids: the structure of vallesiachotamine. apparicine, an indole alkaloid of novel structure. the structure and chemistry of nervobscurine. tubulosine and its chemical correlation with deoxytubulosine, Parts 1–4.
- Schmelzer, G. H. (2008). "Tabernaemontana elegans Stapf". In Schmelzer, G. H.; Gurib-Fakim, A. (eds.). Medicinal Plants 1. Plant Resources of Tropical Africa. Vol. 11. PROTA Foundation; Backhuys Publishers; CTA. pp. 592–593. ISBN 978-90-5782-204-9.
- Shamma, Maurice (1970). "Alkaloids". In Cain, Cornelius K.; et al. (eds.). Annual Reports in Medicinal Chemistry, 1969. Academic Press. pp. 323–332. doi:10.1016/S0065-7743(08)60353-X. ISBN 978-0-12-040505-3.
- Verpoorte, R.; van der Heijden, R.; Schripsema, J.; Sierra, M.; et al. (1989). "Secondary metabolites in cell cultures of Teabernaemontana species". In Kurz, Wolfgang G. W. (ed.). Primary and Secondary Metabolism of Plant Cell Cultures II. Springer-Verlag. pp. 138–148. doi:10.1007/978-3-642-74551-5_16. ISBN 978-3-642-74553-9.
External links
[edit]- (-)-Apparicine in the United States Environmental Protection Agency's CompTox Database
- (-)-Apparicine at KNApSAck
