Trichomonas vaginalis
| Trichomonas vaginalis | |
|---|---|

| |
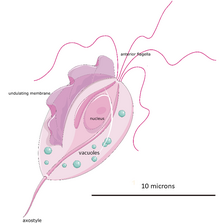
| Trichomonas vaginalis observed by scanning electron microscopy showing the axostyle (ax), the anterior flagella (af) and the undulating membrane (rf).[1] | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Metamonada |
| Order: | Trichomonadida |
| Family: | Trichomonadidae |
| Genus: | Trichomonas |
| Species: | T. vaginalis
|
| Binomial name | |
| Trichomonas vaginalis (Donné 1836)
| |
Trichomonas vaginalis is an anaerobic, flagellated protozoan parasite and the causative agent of a sexually transmitted disease called trichomoniasis. It is the most common pathogenic protozoan that infects humans in industrialized countries.[2] Infection rates in men and women are similar but women are usually symptomatic, while infections in men are usually asymptomatic. Transmission usually occurs via direct, skin-to-skin contact with an infected individual, most often through vaginal intercourse. It is estimated that 160 million cases of infection are acquired annually worldwide.[3] The estimates for North America alone are between 5 and 8 million new infections each year, with an estimated rate of asymptomatic cases as high as 50%.[4] Usually treatment consists of metronidazole and tinidazole.[5]
Clinical
[edit]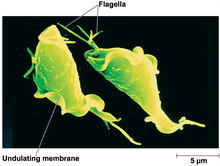
History
[edit]Alfred Francois Donné (1801–1878) was the first to describe a procedure to diagnose trichomoniasis through "the microscopic observation of motile protozoa in vaginal or cervical secretions" in 1836. He published this in the article entitled, "Animalcules observés dans les matières purulentes et le produit des sécrétions des organes génitaux de l'homme et de la femme" in the journal, Comptes rendus de l'Académie des sciences.[6] With it, he created the binomial name of the parasite as Trichomonas vaginalis.[7] 80 years after the initial discovery of the parasitic protozoan, Hohne declared Trichomoniasis as a clinical entity in 1916.[8]
Signs and symptoms
[edit]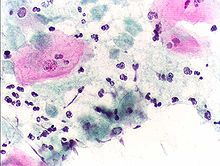
Most women (85%) and men (77%) with infected with T. vaginalis do not have symptoms. Half of these women can develop symptoms within 6 months and can have vaginal erythema, dyspareunia, dysuria, and vaginal discharge, which is often diffuse, malodorous, and yellow-green, along with itching in the genital region. “Strawberry cervix,” occurs in about 5% of women. In men, it can cause urethritis, epididymitis and prostatitis.[9]
Complications
[edit]Some of the complications of Trichomonas vaginalis in women include: preterm delivery, low birth weight, and increased mortality as well as predisposing to human immunodeficiency virus infection, AIDS, and cervical cancer.[10] Trichomonas vaginalis can be seen in diverse locations within the body, such as," in the urinary tract, fallopian tubes, and pelvis and can cause pneumonia, bronchitis, and oral lesions."[11]
Diagnosis
[edit]
Classically, with a cervical smear, infected women may have a transparent "halo" around their superficial cell nucleus but more typically the organism itself is seen with a, "slight cyanophilic tinge, faint eccentric nuclei, and fine acidophilic granules."[12] It is unreliably detected by studying a genital discharge or with a cervical smear because of their low sensitivity. Trichomonas vaginalis is also routinely diagnosed via a wet mount, in which motility is observed. Currently, the most common method of diagnosis is via overnight culture,[13][14] with a sensitivity range of 75–95%.[15] Newer methods, such as rapid antigen testing and transcription-mediated amplification, have even greater sensitivity, but are not in widespread use.[15]
Treatment
[edit]Infection is treated and cured with metronidazole[16] or tinidazole. The CDC recommends a one time dose of 2 grams of either metronidazole or tinidazole as the first-line treatment; the alternative treatment recommended is 500 milligrams of metronidazole, twice daily, for seven days if there is failure of the single-dose regimen.[17] Medication should be prescribed to any sexual partner(s) as well because they may be asymptomatic carriers.[18]
Morphology
[edit]
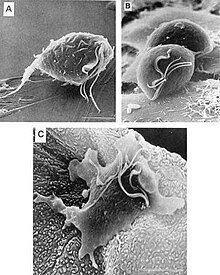
Trichomonas vaginalis exists in only one morphological stage, a trophozoite, and cannot encyst (or form cysts.) This protozoan does not typically adhere to one shape, as in different conditions, the parasite has the ability to change. When in culture separate from the host, it usually displays a more "pear" or oval shaped morphology, but when present in a living host, specifically on the epithelial cells of the vaginal wall, the shape is more "amoeboid".[19] It is slightly larger than a white blood cell, measuring 9 × 7 μm. In both forms, Trichomonas vaginalis has five flagella – four protruding from the front or anterior of the parasite and the fifth on the back or posterior end. The functionality of the fifth flagellum is not known.[20] In addition, a barb-like axostyle projects opposite the four-flagella bundle. All of these flagella are connected to an "undulating" membrane.[20] The axostyle may be used for attachment to surfaces and may also cause the tissue damage seen in trichomoniasis.[21] The nucleus is usually elongated, and is located near the anterior end of the protozoan within the cytoplasm which contains many hydrogenosomes (closed-membrane organelle with the ability to produce both adenosine triphosphate and hydrogen while in anaerobic conditions.)[22]
While Trichomonas vaginalis does not have a cyst form, the organism can survive for up to 24 hours in urine, semen, or even water samples. A nonmotile, round, pseudocystic form with internalized flagella has been observed under unfavorable conditions.[23] This form is generally regarded as a degenerate stage as opposed to a resistant form,[23] although viability of pseudocystic cells has been occasionally reported.[24] The ability to revert to trophozoite form, to reproduce and sustain infection has been described,[25] along with a microscopic cell staining technique to visually discern this elusive form.[26]
Metabolism
[edit]Trichomonas vaginalis is an anaerobe.[27] There is an absence of cytochrome C and mitochondria, thus making oxygen uptake and synthesis of adenosine triphosphate via oxidative phosphorylation difficult.[27] Although it contains no mitochondria, an analogous structure called a hydrogenosome, which is the site of fermentative oxidation of pyruvate, carries out many of the same metabolic processes. Carbohydrates, specifically those with alpha1,4- glycosidic linkages, are metabolized and eventually fermented to produce products such as acetate, lactate, malate, glycerol and CO2 under aerobic conditions. Hydrogen is produced under anaerobic conditions.[28] Outside the hydrogenosome, carbohydrate metabolism also occurs freely in the cytoplasm. The Embden-Meyerhof-Parnas pathway.[28] is used to convert glucose into phosphoenolpyruvate which ultimately becomes pyruvate.
Virulence factors
[edit]Although Trichomonas vaginalis exists as a trophozoite in its infective form, its amoeboid form is also an important characteristic that adds to how well it is able to infect its host. The amoeboid form, which is pancake shaped, allows for greater surface area contact with epithelial cells of the vagina, cervix, urethra, and prostate. [29] The pseudocyst form is also a way in which the microbe can infect more efficiently, but this only induced when exposed to cold and other stressors.[29] These various forms are accompanied with differing protein phosphorylation profiles which are triggered by environmental pressures.[29]
One of the hallmark features of Trichomonas vaginalis is the adherence factors that allow cervicovaginal epithelium colonization in women. The adherence that this organism illustrates is specific to vaginal epithelial cells being pH, time, and temperature dependent.[30] A variety of virulence factors mediate this process some of which are the microtubules, microfilaments, bacterial adhesins (4), and cysteine proteinases. The adhesins are four trichomonad enzymes called AP65, AP51, AP33, and AP23 that mediate the interaction of the parasite to the receptor molecules on vaginal epithelial cells.[31] The best characterized surface molecule associated with one of the four adhesins is called Trichomonas vaginalis lipoglycans.[29] This molecule is the most abundant on the surface of Trichomonas vaginalis, aids in sticking to vaginal epithelial cells, and can also influence how the human immune system responds, affecting inflammatory responses and macrophages in the body.[29] Cysteine proteinases may be another virulence factor because not only do these 30 kDa proteins bind to host cell surfaces but also may degrade extracellular matrix proteins like hemoglobin, fibronectin or collagen IV.[30]
Genome sequencing and statistics
[edit]The Trichomonas vaginalis genome is approximately 160 megabases in size[32] – ten times larger than predicted from earlier gel-based chromosome sizing.[33] (The human genome is ~3.5 gigabases by comparison.[34]) As much as two-thirds of the Trichomonas vaginalis sequence consists of repetitive and transposable elements, indicative of a recent drastic, evolutionarily expansion of the genome. The total number of predicted protein-coding genes is ~60,000, with the genome being around 65% repetitive (virus-like, transposon-like, retrotransposon-like, and unclassified repeats, all with high copy number and low polymorphism).[35] Approximately 26,000 of the protein-coding genes have been classed as 'evidence-supported' (similar either to known proteins, or to expressed sequence tags), while the remainder have no known function. [35]These extraordinary genome statistics are likely to change downward as the genome sequence, currently very fragmented due to the difficulty of ordering repetitive DNA, is assembled into chromosomes, and as more transcription data (expressed sequence tags, microarrays) accumulate. [35]
TrichDB.org was launched as a free, public genomic data repository and retrieval service devoted to genome-scale trichomonad data. The site currently contains all of the Trichomonas vaginalis sequence project data, several expressed sequence tag libraries, and tools for data mining and display. [36] TrichDB is part of the EupathDB functional genomics database project funded by the National Institutes of Health and National Institute of Allergy and Infectious Diseases.[36]
Genetic diversity
[edit]High levels of genetic diversity were detected in Trichomonas vaginalis after phenotypic differences were discovered during clinical presentations. [37] Studies into the genetic diversity of Trichomonas vaginalis has shown that there are two distinct lineages of the parasite found worldwide; both lineages are represented evenly in field isolates. The two lineages differ in whether or not Trichomonas vaginalis virus infection is present. Trichomonas vaginalis virus infection is clinically relevant in that, it has an effect on parasite resistance to metronidazole, a first line drug treatment for human trichomoniasis.[38]
Increased susceptibility to human immunodeficiency virus
[edit]In addition to inflammation that Trichomonas vaginalis causes, the parasite also causes lysis of epithelial cells and red blood cells in the area leading to more inflammation and disruption of the protective barrier usually provided by the epithelium. Having Trichomonas vaginalis also may increase the chances of the infected woman transmitting human immunodeficiency virus to her sexual partner(s).[39][40]
Evolution
[edit]The biology of Trichomonas vaginalis has implications for understanding the origin of sexual reproduction in eukaryotes. Trichomonas vaginalis is not known to undergo meiosis, a key stage of the eukaryotic sexual cycle. However, when Malik et al.[41] examined Trichomonas vaginalis for the presence of 29 genes known to function in meiosis, they found 27 homologous genes to the ones found in animals, fungi, plants and other protists, including eight of nine genes that are specific to meiosis in model organisms.[41] These findings suggest that Trichomonas vaginalis has the capability for meiotic recombination, and hence "parasexual" reproduction.[41] 21 of the 27 meiosis genes were also found in another parasite Giardia lamblia (also called Giardia intestinalis), indicating that these meiotic genes were present in a common ancestor of Trichomonas vaginalis and G. intestinalis.[41] Since these two species are descendants of lineages that are highly divergent among eukaryotes, these meiotic genes were likely present in a common ancestor of all eukaryotes.[41]
See also
[edit]References
[edit]- ^ Dias-Lopes G, Saboia-Vahia L, Margotti ET, Fernandes NS, Castro CL, Oliveira FO, Peixoto JF, Britto C, Silva FC, Cuervo P, Jesus JB (October 2017). "Morphologic study of the effect of iron on pseudocyst formation in Trichomonas vaginalis and its interaction with human epithelial cells". Memórias do Instituto Oswaldo Cruz. 112 (10): 664–673. doi:10.1590/0074-02760170032. PMC 5607515. PMID 28953994.
- ^ Soper, D (2004). "Trichomoniasis: under control or undercontrolled?". American Journal of Obstetrics and Gynecology. 190 (1): 281–90. doi:10.1016/j.ajog.2003.08.023. PMID 14749674.
- ^ Harp, Djana F.; Chowdhury, Indrajit (2011-07-01). "Trichomoniasis: evaluation to execution". European Journal of Obstetrics & Gynecology and Reproductive Biology. 157 (1): 3–9. doi:10.1016/j.ejogrb.2011.02.024. ISSN 0301-2115. PMC 4888369. PMID 21440359.
- ^ Hook, Edward W. (1999). "Trichomonas vaginalis—No Longer a Minor STD". Sexually Transmitted Diseases. 26 (7): 388–9. doi:10.1097/00007435-199908000-00004. PMID 10458631.
- ^ "Trichomonas Vaginalis: Treatment Questions and Challenges". Medscape. Retrieved 2024-11-12.
- ^ Donné, A. (19 September 1836). "Animalcules observés dans les matières purulentes et le produit des sécrétions des organes génitaux de l'homme et de la femme". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences (in French). 3: 385–386.
- ^ Diamantis, Aristidis; Magiorkinis, Emmanouil; Androutsos, George (2009). "Alfred Francois Donné (1801-78): a pioneer of microscopy, microbiology and haematology". Journal of Medical Biography. 17 (2): 81–87. doi:10.1258/jmb.2008.008040. ISSN 0967-7720. PMID 19401511. S2CID 9287263.
- ^ Ryu, Jae-Sook; Min, Duk-Young (2006-06-20). "Trichomonas vaginalis and trichomoniasis in the Republic of Korea". The Korean Journal of Parasitology. 44 (2): 101–116. doi:10.3347/kjp.2006.44.2.101. ISSN 0023-4001. PMC 2532633. PMID 16809958.
- ^ Kissinger PJ, Gaydos CA, Seña AC, Scott McClelland R, Soper D, Secor WE, Legendre D, Workowski KA, Muzny CA (April 2022). "Diagnosis and Management of Trichomonas vaginalis: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines". Clinical Infectious Diseases. 74 (Suppl_2): S152–S161. doi:10.1093/cid/ciac030. PMC 9006969. PMID 35416973.
- ^ Soper, David (2004-01-01). "Trichomoniasis: under control or undercontrolled?". American Journal of Obstetrics and Gynecology. 190 (1): 281–290. doi:10.1016/j.ajog.2003.08.023. ISSN 0002-9378. PMID 14749674.
- ^ Upcroft, Peter; Upcroft, Jacqueline A. (January 2001). "Drug Targets and Mechanisms of Resistance in the Anaerobic Protozoa". Clinical Microbiology Reviews. 14 (1): 150–164. doi:10.1128/CMR.14.1.150-164.2001. ISSN 0893-8512. PMC 88967. PMID 11148007.
- ^ Powers, Celeste N. (1998). "Diagnosis of Infectious Diseases: a Cytopathologist's Perspective". Clinical Microbiology Reviews. 11 (2): 341–65. doi:10.1128/CMR.11.2.341. PMC 106836. PMID 9564567.
- ^ Ohlemeyer, C; Hornberger, L; Lynch, D; Swierkosz, E (March 1998). "Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy". Journal of Adolescent Health. 22 (3): 205–8. doi:10.1016/S1054-139X(97)00214-0. PMID 9502007.
- ^ Sood, Seema; Mohanty, Srujana; Kapil, Arti; Tolosa, Jorge; Mittal, Suneeta (2007). "InPouch TV culture for detection of Trichomonas vaginalis" (PDF). The Indian Journal of Medical Research. 125 (4): 567–71. PMID 17598943.
- ^ a b Huppert, Jill S.; Mortensen, Joel E.; Reed, Jennifer L.; Kahn, Jessica A.; Rich, Kimberly D.; Miller, William C.; Hobbs, Marcia M. (2007). "Rapid Antigen Testing Compares Favorably with Transcription-Mediated Amplification Assay for the Detection of Trichomonas vaginalis in Young Women". Clinical Infectious Diseases. 45 (2): 194–8. doi:10.1086/518851. PMID 17578778.
- ^ "Metronidazole". Drugs.com. Retrieved 23 February 2018.
- ^ Workowski, K.A.; Berman, S.; Centers for Disease Control and Prevention (CDC) (17 December 2010). "Sexually transmitted diseases treatment guidelines, 2010" (PDF). MMWR Recomm Rep. 59 (RR-12): 1–110. PMID 21160459. Erratum in: MMWR Recomm Rep. 2011 Jan 14;60(1):18.
- ^ Cudmore, S. L.; Delgaty, K. L.; Hayward-Mcclelland, S. F.; Petrin, D. P.; Garber, G. E. (2004). "Treatment of Infections Caused by Metronidazole-Resistant Trichomonas vaginalis". Clinical Microbiology Reviews. 17 (4): 783–93, table of contents. doi:10.1128/CMR.17.4.783-793.2004. PMC 523556. PMID 15489348.
- ^ Petrin, Dino; Delgaty, Kiera; Bhatt, Renuka; Garber, Gary (April 1998). "Clinical and Microbiological Aspects of Trichomonas vaginalis". Clinical Microbiology Reviews. 11 (2): 300–317. doi:10.1128/CMR.11.2.300. ISSN 0893-8512. PMC 106834. PMID 9564565.
- ^ a b "CDC - DPDx - Trichomoniasis". www.cdc.gov. 2024-06-07. Retrieved 2024-10-03.
- ^ Ryan, Kenneth James; Ray, C. George; Sherris, John C., eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.[page needed]
- ^ Mercer F, Johnson PJ (August 2018). "Trichomonas vaginalis: Pathogenesis, Symbiont Interactions, and Host Cell Immune Responses". Trends in Parasitology. 34 (8): 683–693. doi:10.1016/j.pt.2018.05.006. PMC 11132421. PMID 30056833.
- ^ a b Petrin D, Delgaty K, Bhatt R, Garber G (April 1998). "Clinical and microbiological aspects of Trichomonas vaginalis". Clin. Microbiol. Rev. 11 (2): 300–17. doi:10.1128/CMR.11.2.300. PMC 106834. PMID 9564565.
- ^ Mielczarek E, Blaszkowska J (November 2015). "Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure". Infection. 44 (4): 447–458. doi:10.1007/s15010-015-0860-0. PMID 26546373. S2CID 33823038.
- ^ Hussein EM, Atwa MM (December 2008). "Infectivity of Trichomonas vaginalis pseudocysts inoculated intra-vaginally in mice". Journal of the Egyptian Society of Parasitology. 38 (3): 749–762. PMID 19209760.
- ^ Khatoon, Razia (2014). "Evaluation of Different Staining Techniques in the Diagnosis of Trichomonas vaginalis Infection in Females of Reproductive Age Group". Journal of Clinical and Diagnostic Research. 8 (12): DC05-8. doi:10.7860/JCDR/2014/9765.5261. PMC 4316250. PMID 25653944.
- ^ a b Leitsch, David (4 March 2021). "Recent advances in the molecular biology of the protist parasite Trichomonas vaginalis". Faculty Reviews. 10 (26): 26. doi:10.12703/r/10-26. PMC 7946386. PMID 33718943.
- ^ a b Petrin, Dino; Delgaty, Kiera; Bhatt, Renuka; Garber, Gary (1998). "Clinical and Microbiological Aspects of Trichomonas vaginalis". Clinical Microbiology Reviews. 11 (2): 300–317. doi:10.1128/cmr.11.2.300. PMC 106834. PMID 9564565.
- ^ a b c d e Hirt RP (September 2013). "Trichomonas vaginalis virulence factors: an integrative overview". Sexually Transmitted Infections. 89 (6): 439–43. doi:10.1136/sextrans-2013-051105. PMC 3749517. PMID 23694938.
- ^ a b Mendoza-Lopez, M. R.; Becerril-Garcia, C.; Fattel-Facenda, L. V.; Avila-Gonzalez, L.; Ruiz-Tachiquin, M. E.; Ortega-Lopez, J.; Arroyo, R. (2000). "CP30, a Cysteine Proteinase Involved in Trichomonas vaginalis Cytoadherence". Infection and Immunity. 68 (9): 4907–12. doi:10.1128/IAI.68.9.4907-4912.2000. PMC 101697. PMID 10948104.
- ^ Arroyo, R.; Engbring, J.; Alderete, J. F. (1992). "Molecular basis of host epithelial cell recognition by Trichomonas vaginalis". Molecular Microbiology. 6 (7): 853–862. doi:10.1111/j.1365-2958.1992.tb01536.x. PMID 1602965. S2CID 44364966.
- ^ Carlton, J. M.; Hirt, R. P.; Silva, J. C.; Delcher, A. L.; Schatz, M.; Zhao, Q.; Wortman, J. R.; Bidwell, S. L.; et al. (2007). "Draft Genome Sequence of the Sexually Transmitted Pathogen Trichomonas vaginalis". Science. 315 (5809): 207–12. Bibcode:2007Sci...315..207C. doi:10.1126/science.1132894. PMC 2080659. PMID 17218520.
- ^ Lehker, M. W.; Alderete, J. F. (1999). "Resolution of Six Chromosomes of Trichomonas vaginalis and Conservation of Size and Number among Isolates". The Journal of Parasitology. 85 (5): 976–9. doi:10.2307/3285842. JSTOR 3285842. PMID 10577741.
- ^ Zimmer, C. (2007). "EVOLUTION: Jurassic Genome". Science. 315 (5817): 1358–9. doi:10.1126/science.315.5817.1358. PMID 17347424. S2CID 34154189.
- ^ a b c Carlton, Jane M.; Hirt, Robert P.; Silva, Joana C.; Delcher, Arthur L.; Schatz, Michael; Zhao, Qi; Wortman, Jennifer R.; Bidwell, Shelby L.; Alsmark, U. Cecilia M.; Besteiro, Sébastien; Sicheritz-Ponten, Thomas; Noel, Christophe J.; Dacks, Joel B.; Foster, Peter G.; Simillion, Cedric (2007-01-12). "Draft Genome Sequence of the Sexually Transmitted Pathogen Trichomonas vaginalis". Science. 315 (5809): 207–212. Bibcode:2007Sci...315..207C. doi:10.1126/science.1132894. ISSN 0036-8075. PMC 2080659. PMID 17218520.
- ^ a b Aurrecoechea, Cristina; Brestelli, John; Brunk, Brian P.; Carlton, Jane M.; Dommer, Jennifer; Fischer, Steve; Gajria, Bindu; Gao, Xin; et al. (2009). "GiardiaDB and TrichDB: integrated genomic resources for the eukaryotic protist pathogens Giardia lamblia and Trichomonas vaginalis". Nucleic Acids Research. 37 (Database issue): D526–30. doi:10.1093/nar/gkn631. PMC 2686445. PMID 18824479.
- ^ Squire, Daniel S.; Lymbery, Alan J.; Walters, Jennifer; Brigg, Frances; Paparini, Andrea; Thompson, R. C. Andrew (2020-08-01). "Population structure and genetic diversity of Trichomonas vaginalis clinical isolates in Australia and Ghana". Infection, Genetics and Evolution. 82: 104318. Bibcode:2020InfGE..8204318S. doi:10.1016/j.meegid.2020.104318. ISSN 1567-1348. PMID 32278146.
- ^ Conrad, Melissa D.; Gorman, Andrew W.; Schillinger, Julia A.; Fiori, Pier Luigi; Arroyo, Rossana; Malla, Nancy; Dubey, Mohan Lal; Gonzalez, Jorge; Blank, Susan (2012). "Extensive Genetic Diversity, Unique Population Structure and Evidence of Genetic Exchange in the Sexually Transmitted Parasite Trichomonas vaginalis". PLOS Neglected Tropical Diseases. 6 (3): e1573. doi:10.1371/journal.pntd.0001573. PMC 3313929. PMID 22479659.
- ^ Mulla, Summaiyaa; Kosambiya, JK; Desai, Vikask; Shethwala, Nimishad (2009). "Sexually transmitted infections and reproductive tract infections in female sex workers". Indian Journal of Pathology and Microbiology. 52 (2): 198–9. doi:10.4103/0377-4929.48916. PMID 19332911.
- ^ Mavedzenge, Sue Napierala; Van der Pol, Barbara; Cheng, Helen; Montgomery, Elizabeth T.; Blanchard, Kelly; de Bruyn, Guy; Ramjee, Gita; Van der Straten, Ariane (2010). "Epidemiological Synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African Women". Sexually Transmitted Diseases. 37 (7): 460–6. doi:10.1097/OLQ.0b013e3181cfcc4b. PMID 20562586. S2CID 19708165.
- ^ a b c d e Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM (2008). "An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis". PLOS ONE. 3 (8): e2879. Bibcode:2008PLoSO...3.2879M. doi:10.1371/journal.pone.0002879. PMC 2488364. PMID 18663385.
Further reading
[edit]- Hernández, Hilda M.; Marcet, Ricardo; Sarracent, Jorge (28 October 2014). "Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis". Parasite. 21 (54): 54. doi:10.1051/parasite/2014054. PMC 4209856. PMID 25348828.
External links
[edit]- TIGR's Trichomonas vaginalis genome sequencing project.
- TrichDB: the Trichomonas vaginalis genome resource
- NIH site on trichomoniasis.
- Taxonomy
- eMedicine article on trichomoniasis.
- Patient UK
