Transition metal sulfito complex

Transition metal sulfito complexes are coordination compounds containing sulfite (SO32-) as a ligand. The inventory is large. Few sulfito complexes have commercial applications, but sulfite is a substrate for the molybdoenzyme sulfite oxidase.[2]
Bonding modes
[edit]In principle, sulfite can bond to metal ions via S or O. To some extent, the sulfito ligand resembles nitrito (NO−2), which can bind through N or O. Monodentate, S-bonded sulfites are more common than O-bonded sulfito ligands. S-Bonded sulfite is a soft ligand with a strongly trans labilizing effect as indicated by the rapid aquation of [Co(SO3)(NH3)5]+ to give [Co(SO3)(NH3)4(H2O)]+.[3]
In some cases, sulfite serves as a bridging ligand forming M-SO2-O-M’ linkages.[4]
Examples
[edit]- [Co(tetren)SO3]+[5] (tetren = HN(CH2CH2NHCH2CH2NH2)2)
- [M(SO3)2(en)2]− (M = Rh,[6] Co[7][8], en = ethylenediamine)
- [Pd(en)(SO3)2]2− [9]
- [Au(en)(SO3)2]− [10]
Complexes with modified sulfito ligands
[edit]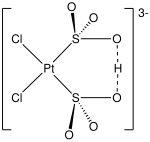
Being dibasic, sulfito ligands are susceptible to O-alkylation and O-protonation. Some examples:
References
[edit]- ^ I. Bernal; J. Cetrullo; W. G. Jackson (1993). "The Phenomenon of Conglomerate Crystallization in Coordination Compounds. XXIII: The Crystallization Behavior of [cis-Co(en)2(N3)(SO3)]·2H2O (I) and of [cis-Co(en)2(NO2)(SO3)]·H2O (II)". Struct. Chem. 4: 235. doi:10.1007/BF00673698. S2CID 94847897.
- ^ Enemark, John H.; Cooney, J. Jon A.; Wang, Jun-Jieh; Holm, R. H. (2004). "Synthetic Analogues and Reaction Systems Relevant to the Molybdenum and Tungsten Oxotransferases". Chemical Reviews. 104 (2): 1175–1200. doi:10.1021/cr020609d. PMID 14871153.
- ^ Halpern, Jack; Palmer, Richard A.; Blakley, Lynne M. (1966). "Substitution Reactions of Sulfitopentaamminecobalt(III) and Its Derivatives. Evidence for a Limiting SN1 Mechanism". Journal of the American Chemical Society. 88 (12): 2877–2878. Bibcode:1966JAChS..88.2877H. doi:10.1021/ja00964a063.
- ^ Krieglstein, Roland; Breitinger, Dietrich K.; Liehr, Günther (2001). "Structural Investigations of Sulfite-Bridged Binuclear Complexes of Platinum(II) and Palladium(II)". European Journal of Inorganic Chemistry (12): 3067–3072. doi:10.1002/1099-0682(200112)2001:12<3067::AID-EJIC3067>3.0.CO;2-8.
- ^ Schneider, K.J.; Van Eldik, R.; Roodt, A.; Leipoldt, J.G. (1986). "The Formation and Reactivity of Sulfito Complexes of Tetraethylenepentamine-Cobalt(III): X-Ray Structure of αα-[co(tetren)SO3]ClO4". Inorganica Chimica Acta. 122: 1–5. doi:10.1016/S0020-1693(00)81258-X.
- ^ Petrikowski, G.; Breitinger, D. K. (1985). "Sodium trans-Bis(1,2-ethanediamine)disulfitorhodate(III) Trihydrate, Na[Rh(C2H8N2)2(SO3)2].3H2O". Acta Crystallographica Section C Crystal Structure Communications. 41 (4): 522–525. doi:10.1107/S0108270185004504.
- ^ Raston, CL; White, AH; Yandell, JK (1979). "Crystal Structure of Disodium(I) cis-Bis(ethane-1,2-diamine)disulfitocobaltate(III) Perchlorate Trihydrate". Australian Journal of Chemistry. 32 (2): 291. doi:10.1071/CH9790291.
- ^ Hargens, Robert D.; Min, Woonza; Henney, Robert C. (1973). Bis(ethylenediamine)sulfito Complexes of Cobalt(III). Inorganic Syntheses. Vol. 14. pp. 77–81. doi:10.1002/9780470132456.ch15. ISBN 978-0-470-13174-9.
- ^ Breitinger, D. K.; Schottner, G.; Raidel, M.; Beck, H. P. (1986). "Kristallstruktur und Schwingungsspektrometrische Daten von cis-Na2[Pd(SO3)2en]·4H2O". Zeitschrift für Anorganische und Allgemeine Chemie. 539 (8): 18–26. doi:10.1002/zaac.19865390803.
- ^ Dunand, A.; Gerdil, R. (1975). "The Crystal and Molecular Structure of Ethylenediammonium bis{ cis -[ethylenediaminedisulphitoaurate(III)]}". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 31 (2): 370–374. Bibcode:1975AcCrB..31..370D. doi:10.1107/S0567740875002828.
- ^ a b Kehr, W. G.; Breitinger, D. K.; Bauer, G. (1980). "Tripotassium cis -dichloro(hydrogendisulfito)platinate(II) K3[Pt{(SO3)2H}Cl2]. A Case of an Extremely Short Hydrogen Bond O...H...O". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 36 (11): 2545–2550. doi:10.1107/S0567740880009405.
- ^ Allen, L. R.; Jeter, D. Y.; Cordes, A. W.; Durham, B. (1988). "(Polypyridine)ruthenium(II) Complexes Containing Sulfite, Bisulfite, and Sulfur Dioxide". Inorganic Chemistry. 27 (22): 3880–3885. doi:10.1021/ic00295a003.
- ^ Hughes, Glyn R.; Minshall, Peter C.; Mingos, D. Michael P. (1979). "The Crystal and Molecular Structure of trans-Di(methylsulphito)bis-(Triphenylphosphine)platinum(II)". Transition Metal Chemistry. 4 (3): 147–150. doi:10.1007/BF00619056.
- ^ Krieglstein, Roland; Breitinger, Dietrich K.; Liehr, Günther (2001). "Structural Investigations of Sulfite-Bridged Binuclear Complexes of Platinum(II) and Palladium(II)". European Journal of Inorganic Chemistry (12): 3067–3072. doi:10.1002/1099-0682(200112)2001:12<3067::AID-EJIC3067>3.0.CO;2-8.
