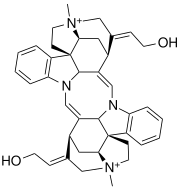
Toxiferine
 | |
| Clinical data | |
|---|---|
| Other names | C-Toxiferine I
C-Toxiferin I Toxiferine I |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C40H46N4O2 |
| Molar mass | 614.834 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Toxiferine, also known as c-toxiferine I, is one of the most toxic plant alkaloids known. It is derived from several plant species, including Strychnos toxifera and Chondrodendron tomentosum. Historically, it has been used as an arrow poison by indigenous peoples in South America for its neuromuscular blocking properties, allowing them to paralyze animals during hunting, but also possibly kill due to paralysis of the respiratory muscles.[1] Toxiferine functions as an acetylcholine receptor (AChR) antagonist. The paralysis caused by toxiferine can in turn be antagonized by neostigmine.[2]
Toxiferine is the most important component in calabash curare. Curare poisons contain many different toxins with similar properties of toxiferine. The most well known component of curare is tubocurarine. The paralysis caused by toxiferine is very similar to that caused by tubocurarine, however toxiferine is ~170 times as potent.[3] The preparation of curare poisons involves complex rituals wherein the tribes extract toxins from various plants.
History
[edit]Curare was discovered in 1595, however toxiferine was only first isolated and characterized in 1941 by Wieland, Bähr and Witkop.[4] They managed to produce only a couple micrograms of this compound as it is quite hard to isolate in large enough quantities to study. This is due to the complexity of curare as it is composed of many different alkaloids. In 1949 King was able to isolate 12 different types of toxiferines (I to XII).[5] In 1951 some of these toxiferine types were analyzed for their toxicological and pharmacological properties. These types were found to differ slightly in structure and their potency.[3]
Curares like tubocurarine were later used as anesthetics in medical procedures, but were replaced in the 1960s by synthetic curare-like drugs like alcuronium, pancuronium, atracurium and vecuronium. These drugs were safer to use as they had a shorter duration of action and less side effects.[6]
After the replacement of curares by these synthetic alternatives, research on toxiferine declined as it was hard to isolate from calabash curare and better alternatives to curares had been found thus decreasing the interest in researching this specific compound. However, curares as a whole have been (and still are) extensively researched.[6]
Use/purpose
[edit]Toxiferine is most commonly known for its use as an arrow poison alongside other curares by south american tribes. It is extracted from plants, like strychnos toxifera and chondrodendron tomentosum. It is hard to extract in large quantities. It is very toxic however, so small quantities will suffice in paralyzing or killing animals while hunting.[3] It could be used as an anesthetic in medical procedures, however it has a very long duration of action which doesn't make it suitable for such procedures. It is also unstable in solution, which further prevents its use in medical settings. Synthetic alternatives like alcuronium can and are still used in anesthetics due to their relatively shorter duration of action and fewer side effects.[7]
Efficacy
[edit]Toxiferine is especially useful as an arrow poison because of its very minimal absorption through oral ingestion. Which is why it is safe to eat the animal after it has been shot with an arrow covered in toxiferine. It is also believed that because of its activity as muscle paralyzer, it can retain glycogen and ATP from releasing after death and by this delay rigor mortis. This makes the meat more tender for longer and maintains its flavor.[8]
Mechanism of action
[edit]Toxiferine I competes with acetylcholine, a neurotransmitter, for binding to the nicotinic acetylcholine receptors on the post-synaptic membrane of the neuromuscular junction. By binding to these receptors, toxiferine I prevents acetylcholine from attaching to them. This inhibition blocks the ion channels associated with these receptors from opening, thereby preventing the influx of sodium ions into the muscle cell. The prevention of sodium influx leads to an inhibition of depolarization of the post-synaptic membrane, which is a necessary step for muscle contraction. Without depolarization, the muscle fiber cannot generate an action potential, resulting in muscle paralysis.
Toxiferine I is a potent antagonist for several acetylcholine receptors, but especially potent for muscle-type nAChR:[9]
| Compound | nicotinic acetylcholine receptor (muscle-type nAChR) Ki (nM) |
alpha-7 nicotinic receptor (α7 nAChR) IC50 (nM) |
muscarinic acetylcholine receptor M2 (allosteric site) EC0.5,diss (nM) | |||
|---|---|---|---|---|---|---|
| toxiferine I | 14 | 9500 | 96 | |||
| alcuronium | 234 | 4100 | 2 | |||
Binding
[edit]Similar to alcuronium, toxiferine is classified as a non-depolarizing neuromuscular-blocking drug. These are drugs that inhibit signal transduction by competitive inhibition of in this case mainly muscle-type nAChRs.[10] Toxiferine though binds 17 times stronger to muscle-type nAChRs than its pharmacological analogue alcuronium.[9] The quaternary ammonium salt that toxiferine and its analogues share with acetylcholine is thought to be the reason for the binding affinity to the AChRs. The exact reason for the especially high binding affinity of toxiferine to for example muscle-type nAChRs is unknown. There have been attempts at understanding the exact binding of toxiferine in nAChRs, but the models are dated.[11][12]
Reversing the mechanism
[edit]Neostigmine is known to be effective at reversing the competitive inhibition of toxiferine and its analogues.[13] Neostigmine works by inhibiting acetylcholinesterase, increasing the acetylcholine concentrations so it can compete more with the non-depolarizing neuromuscular-blocking drug. By this toxiferine can be freed into the circulation for excretion.[14]
Chemistry
[edit]Structure
[edit]Toxiferine I is an indole alkaloid derived from tryptamine. It has a dimeric structure with each monomer containing a quaternary ammonium salt. The parent structure, without counter ions, has the molecular formula C40H46N4O22+,[15] while the dichloride salt has the molecular formula C40H46N4O2Cl2. Alkaloids are naturally occurring compounds that are basic and contain at least one nitrogen atom.[16] Toxiferine is classified as a dimeric bisindole alkaloid because it is symmetrically constructed from two identical monomeric units, each containing an indole ring.[17][18]
Analogues
[edit]A dozen different types of toxiferine were found to exist (designated as toxiferine I to XII),[5] but the different structures of toxiferine II till XII have not been studied in great detail. Toxiferine does have multiple analogues which are researched extensively. Some of these include: bisnortoxiferine, caracurine V and alcuronium. Caracurine V is, like toxiferine, another naturally occurring curare toxin from the strychnos toxifera.[19] Caracurine V, unlike the other analogues, has a closed ring formed between the hydroxyl groups and the middle ring. Though the main difference between the analogues are the side groups attached to the positive nitrogen atom, also called the quaternary ammonium ion. The main target of toxiferine and its analogues are acetylcholine receptors. It is this quaternary ammonium ion that both toxiferine and its analogues share with acetylcholine that gives them their specific affinity for these receptors. The difference in hydroxyl side groups together with most importantly the quaternary ammonium side groups is believed to cause the variability in receptor affinity and metabolic activity and with this a variability in the toxic properties of these analogues.[9] From this it can be concluded that the side groups of toxiferine, mainly those attached to the quaternary ammonium have large effect over its reactivity and affinity, but to this day no research exists that can reliably explain the structural reactivity of toxiferine.
Biosynthesis
[edit]Toxiferine is an indole alkaloid. Indole alkaloids are the largest among the alkaloids. They are primarily synthesized using tryptophan.[20][21] The dimeric subunits of toxiferine bear high similarity to strychnine and may be a related product of its biosynthesis. Especially the intermediate Wieland-Gumlich aldehyde is very similar to the dimeric subunits of toxiferine, though this lacks the quaternary ammonium ion. The biosynthesis of strychnine was solved in 2022.[22] It is also possible to artificially synthesise strychnine. The exact biosynthetic and possible artificial ways to synthesise toxiferine are not known.
It is believed though that toxiferine may be biosynthetically derived from the monoterpenoid indole alkaloid strictosidine. Strictosidine in turn is derived from the alkaloid tryptamine and the terpene secologanin through the action of strictosidine synthase.[23]
ADME
[edit]This describes the ADME of toxiferine.
Absorption
[edit]Toxiferine is known to enter the body in two different ways: either orally by ingestion or intravenously while applied to a sharp tip for killing purposes. When orally ingested, toxiferine is only absorbed minimally into the plasma and is not known to be dangerous even though it has a very high potency. Intravenous absorption is thus the only way of effective administration.[6]
Distribution
[edit]The distribution of toxiferine has been researched in rats. Toxiferine distributes through the body in a way that is similar to other non-depolarizing curare alkaloids. Toxiferine is a highly water soluble substance and because of this also generally not lipophilic. Toxiferine does not easily pass the blood-brain barrier. It is mostly retained in motor endplates and the sciatic nerve where it binds to specific receptors. It was also found that toxiferine is distributed to tissues with a high acidic mucopolysaccharide content like intervertebral discs and cartilage of the ribs.[24] Alkaloids are known to have affinity for such polysaccharides at acidic extracellular pH.[25]
Metabolism
[edit]Toxiferine does not have any known metabolic pathways. This may be due to its low lipophilicity. Bis-quaternary nitrogen compounds like toxiferine have shown to be dependent on their lipophilicity to be transported to sites in the liver.[26] The liver, which is the main site of metabolic activity, can thus not or hardly be reached. When comparing the metabolic activity of toxiferine to that of maybe its most important analogue alcuronium, it is observed that the allylic side chains of alcuronium make it more potent for biotransformation than toxiferine with its methyl side chains. This makes the duration of action of toxiferine significantly longer when compared to alcuronium.[27]
Excretion
[edit]Toxiferine is mainly excreted in the urine even though elimination of toxiferine by the kidney is very poor relative to its analogue alcuronium. Toxiferine has a strong receptor affinity, which together with the poor excretion makes it accumulate in the body rapidly after repeated administration.[24][28] This is another reason why toxiferine has an especially long duration of action in the body.
Toxicological data
[edit]The effects of toxiferine have been studied in multiple organisms like rhesus monkeys, guinea pigs and mice by intravenous (IV) and intramuscular (IM) injections of different doses of toxiferine. The data on rhesus monkeys likely resembles human effects more closely.
| ED50 (μg/kg) | LD50 (μg/kg) | Therapeutic index (LD50/ED50) | Margin of safety (LD1/ED99) | Time till onset (min) | |
|---|---|---|---|---|---|
| IV | 5.5 | 8.9 | 1.61 | 1.33 | <5 |
| IM | 6.5 | 17.8 | 2.74 | 1.89 | <15 |
It has to be said that the duration of paralysis varies a lot between different individual monkeys and doses. It could be as short as 6 minutes but also as long as 85 minutes.[29] In mice the LD100 was determined to be 23 μg/kg with a duration of paralysis around 12 minutes.[7]
References
[edit]- ^ Mitsunaga T (2023). "The Science of Useful Plants in the Central Andes Area in South America IX: The Herbs in the Alkaloid Region 1". Foods & Food Ingredients Journal of Japan. 228 (4): 336–350. doi:10.34457/ffij.228.4_336.
- ^ Saxton JE, Gorman AA, Hesse M, Schmid H, Waser PG, Hopff WH (1971). "Bisindole alkaloids". In Saxton JE (ed.). The Alkaloids: v. 1: A Review of Chemical Literature. Specialist Periodical Reports. Cambridge, England: Royal Society of Chemistry. p. 330. ISBN 0-85186-257-8.
- ^ a b c Paton WD, Perry WL (June 1951). "The pharmacology of the toxiferines". British Journal of Pharmacology and Chemotherapy. 6 (2): 299–310. doi:10.1111/j.1476-5381.1951.tb00643.x. PMC 1509221. PMID 14848460.
- ^ Wieland H, Bähr I, Witkop B (1941). "About the alkaloides from calebash-curare IV". Justus Liebigs Annalen der Chemie. 547: 156–179. doi:10.1002/jlac.19415470111.
- ^ a b King H (1949). "684. Curare alkaloids. Part X. Some alkaloids of Strychnos toxifera Rob. Schomb". Journal of the Chemical Society (Resumed): 3263–3271. doi:10.1039/jr9490003263. ISSN 0368-1769.
- ^ a b c Bowman WC (January 2006). "Neuromuscular block". British Journal of Pharmacology. 147 (Suppl 1): S277 – S286. doi:10.1038/sj.bjp.0706404. PMC 1760749. PMID 16402115.
- ^ a b Philippe G, Angenot L, Tits M, Frédérich M (September 2004). "About the toxicity of some Strychnos species and their alkaloids". Toxicon. 44 (4): 405–416. Bibcode:2004Txcn...44..405P. doi:10.1016/j.toxicon.2004.05.006. PMID 15302523.
- ^ Bowman WC (1986). Mechanisms of Action of Neuromuscular Blocking Drugs. London: Palgrave Macmillan. pp. 65–96. ISBN 978-1-349-08028-1.
- ^ a b c Zlotos DP, Tränkle C, Holzgrabe U, Gündisch D, Jensen AA (September 2014). "Semisynthetic analogues of toxiferine I and their pharmacological properties at α7 nAChRs, muscle-type nAChRs, and the allosteric binding site of muscarinic M2 receptors". Journal of Natural Products. 77 (9): 2006–2013. doi:10.1021/np500259j. PMC 4176391. PMID 25192059.
- ^ Clar DT, Liu M (2024), "Nondepolarizing Neuromuscular Blockers", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30521249, retrieved 2024-06-30
- ^ Smythies JR (May 1971). "The molecular nature of the acetylcholine receptor: a stereochemical study". European Journal of Pharmacology. 14 (3): 268–279. doi:10.1016/0014-2999(71)90136-1. PMID 5156145.
- ^ Smythies JR (September 1980). "An hypothesis concerning the molecular structure of the nicotinic acetylcholine receptor". Medical Hypotheses. 6 (9): 943–950. doi:10.1016/0306-9877(80)90046-8. PMID 7432253.
- ^ Stovner J, Theodorsen L, Bjelke E (April 1972). "Sensitivity to dimethyltubocurarine and toxiferine with special reference to serum proteins". British Journal of Anaesthesia. 44 (4): 374–380. doi:10.1093/bja/44.4.374. PMID 5032074.
- ^ Pollard BJ (June 2005). "Neuromuscular blocking agents and reversal agents". Anaesthesia & Intensive Care Medicine. 6 (6): 189–192. doi:10.1383/anes.6.6.189.65784. ISSN 1472-0299.
- ^ "Toxiferine". PubChem. U.S. National Library of Medicine. Retrieved 2024-06-27.
- ^ Moss GP, Smith P, Tavernier D (January 1995). "Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995)". Pure and Applied Chemistry (in German). 67 (8–9): 1307–1375. doi:10.1351/pac199567081307. ISSN 1365-3075.
- ^ Plemenkov VV (2001). "Introduction to the Chemistry of Natural Compounds". Kazan: 242.
- ^ Hesse M (2002). Alkaloids. Nature's Curse or Blessing?. Zürich: VHCA, Wiley-VCH. ISBN 3-906390-24-1.
- ^ Zlotos DP, Gündisch D, Ferraro S, Tilotta MC, Stiefl N, Baumann K (December 2004). "Bisquaternary caracurine V and iso-caracurine V salts as ligands for the muscle type of nicotinic acetylcholine receptors: SAR and QSAR studies". Bioorganic & Medicinal Chemistry. 12 (23): 6277–6285. doi:10.1016/j.bmc.2004.08.053. PMID 15519170.
- ^ "C-Toxiferin I". roempp.thieme.de (in German). Retrieved 2024-06-28.
- ^ Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, et al. (July 2017). "Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements". EFSA Journal. 15 (7): 505–567. doi:10.1016/B978-0-12-816455-6.00015-9. PMC 7153348.
- ^ Hong B, Grzech D, Caputi L, Sonawane P, López CE, Kamileen MO, et al. (July 2022). "Biosynthesis of strychnine". Nature. 607 (7919): 617–622. Bibcode:2022Natur.607..617H. doi:10.1038/s41586-022-04950-4. PMC 9300463. PMID 35794473.
- ^ Stöckigt J, Panjikar S (December 2007). "Structural biology in plant natural product biosynthesis--architecture of enzymes from monoterpenoid indole and tropane alkaloid biosynthesis". Natural Product Reports. 24 (6): 1382–400. doi:10.1039/b711935f. PMID 18033585.
- ^ a b Waser PG, Reller J (June 1972). "Distribution and pharmacokinetics of 14 C-toxiferine in rats". Agents and Actions. 2 (4): 170–175. doi:10.1007/BF01965855. PMID 5041048.
- ^ Zsila F (21 December 2015). "The anticancer agent ellipticine binds to glycosaminoglycans at mildly acidic pH characteristic of the extracellular matrix of tumor tissues". RSC Advances. 6 (1): 810–814. doi:10.1039/C5RA23437A. ISSN 2046-2069.
- ^ Meijer DK, Weitering JG (May 1970). "Curare-like agents: relation between lipid solubility and transport into bile in perfused rat liver". European Journal of Pharmacology. 10 (2): 283–289. doi:10.1016/0014-2999(70)90284-0. PMID 4245935.
- ^ Ariëns EJ (1971). Drug design. Medicinal chemistry. New York: Academic press. ISBN 978-0-12-060301-5.
- ^ Frey R, Seeger R (March 1961). "Experimental and clinical experience with toxiferine (alkaloid of calabash curare)". Canadian Anaesthetists' Society Journal. 8 (2): 99–117. doi:10.1007/BF03021339. PMID 13701839.
- ^ Rosato RR, Stephen EL, Pannier WL (January 1976). "Dose-response data for toxiferine dichloride in monkeys and guinea pigs". Toxicology and Applied Pharmacology. 35 (1): 107–111. Bibcode:1976ToxAP..35..107R. doi:10.1016/0041-008x(76)90115-0. PMID 816038.
