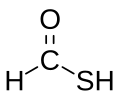
Thioformic acid
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Methanethioic S-acid
| |
| Other names
Monothioformic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH2OS | |
| Molar mass | 62.09 g·mol−1 |
| Boiling point | 86 °C (187 °F; 359 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thioformic acid is a chemical compound with the molecular formula CH2OS. As the simplest thiocarboxylic acid,[2] it has been the subject of studies on its properties which may be extrapolated to more complex thiocarboxylic acids.[3][4][5]
Like other thiocarboxylic acids, thioformic acid can occur in either of two tautomers. The ratio of the two is dependent on solvent and other factors.[6][7]
Thioformic acid has been detected in the interstellar medium near the giant molecular cloud G+0.693–0.027 and the hot core G31.41+0.31.[2][5][8]
References
[edit]- ^ Adams, E. P.; Ayad, K. N.; Doyle, F. P.; Holland, D. O.; Hunter, W. H.; Nayler, J. H. C.; Queen, A. (1960). "Antituberculous sulfur compounds. III. Substituted propylene sulfides". Journal of the Chemical Society: 2665–2673. doi:10.1039/jr9600002665.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wang, Jia; Marks, Joshua H.; Tuli, Lotefa B.; Mebel, Alexander M.; Azyazov, Valeriy N.; Kaiser, Ralf I. (2022). "Formation of Thioformic Acid (HCOSH)─The Simplest Thioacid─in Interstellar Ice Analogues". The Journal of Physical Chemistry A. 126 (51): 9699–9708. Bibcode:2022JPCA..126.9699W. doi:10.1021/acs.jpca.2c06860. PMID 36534075.
- ^ Williams, Gwilym A.; MacDonald, John N.; Boggs, James E. (1990). "Comment on the geometries of cis and trans thioformic and formic acids". Journal of Molecular Structure. 220 (1–2): 321–326. Bibcode:1990JMoSt.220..321W. doi:10.1016/0022-2860(90)80122-Z.
- ^ Raaska, T. (1989). "Ab initio SCF studies on thioformic acid: Basis-set effects". Journal of Molecular Structure: Theochem. 201 (1–2): 59–67. doi:10.1016/0166-1280(89)87062-9.
- ^ a b García de la Concepción, J.; Colzi, L.; Jiménez-Serra, I.; Molpeceres, G.; Corchado, J. C.; Rivilla, V. M.; Martín-Pintado, J.; Beltrán, M. T.; Mininni, C. (2022). "The trans/Cis ratio of formic (HCOOH) and thioformic (HC(O)SH) acids in the interstellar medium". Astronomy & Astrophysics. 658: A150. arXiv:2111.10842. Bibcode:2022A&A...658A.150G. doi:10.1051/0004-6361/202142287.
- ^ Delaere, David; Raspoet, Greet; Nguyen, Minh Tho (1999). "Thiol−Thione Tautomerism in Thioformic Acid: Importance of Specific Solvent Interactions". The Journal of Physical Chemistry A. 103 (1): 171–177. Bibcode:1999JPCA..103..171D. doi:10.1021/jp983298c.
- ^ Duarte, Fernanda; Toro-Labbé, Alejandro (2010). "The catalytic effect of water on the keto-enol tautomerisation reaction of thioformic acid". Molecular Physics. 108 (10): 1375–1384. Bibcode:2010MolPh.108.1375D. doi:10.1080/00268971003698064.
- ^ Rodríguez-Almeida, Lucas F.; Jiménez-Serra, Izaskun; Rivilla, Víctor M.; Martín-Pintado, Jesús; Zeng, Shaoshan; Tercero, Belén; De Vicente, Pablo; Colzi, Laura; Rico-Villas, Fernando; Martín, Sergio; Requena-Torres, Miguel A. (2021). "Thiols in the Interstellar Medium: First Detection of HC(O)SH and Confirmation of C2H5SH". The Astrophysical Journal Letters. 912 (1): L11. arXiv:2104.08036. Bibcode:2021ApJ...912L..11R. doi:10.3847/2041-8213/abf7cb.

