Talk:Glow discharge
| This article is rated C-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||||||||||||
| |||||||||||||||||||||
Comments
[edit]Can somebody write how to get a long lifespan of the cathode, please!
- thermionic emission leads to evaporation
- secondary electrons lead to sputter
- The plasma globe does not need any cathode, so why is not any discharge lamped operated in this way?
--Arnero 09:25, 22 June 2006 (UTC)
- I assume you're referring to the fact that a plasma lamp's electrode is basically capacitively coupled to the gas mixture (and so, doesn't exactly emit any electrons itself)? I think you'll find that the factor that limits wider application is that the value (capacitance) of the "coupling capacitor" is too small to permit much power to pass through it unless the operating frequency were impractically high. So plasma lamps just emit a gentle glow and not much actual light.
- You might also want to look at sulfur lamps and electrodeless lamps or induction lamps or whaever they've named those articles this week. That's a much highe-powered application of the sort of thing you're talking about, but in the case of the sulfur lamp, the lamp comes along with a serious radio frequency interference problem as it tries to couple a kilowatt or so of power into the gas.
- Atlant 11:26, 22 June 2006 (UTC)
- in reference to "Can somebody write how to get a long lifespan of the cathode, please" - No one should, wiki is not a how to 71.195.237.212 (talk) 18:35, 8 February 2008 (UTC)
- One way to get longer life is to get rid of the cathode altogether. That's how electrodeless induction lamps work - the discharge tube is a loop forming the secondary winding of a transformer, so it has no electrodes to wear out. Then the lamp life is governed by the life of the phosphor coating, and/or loss of mercury into the phosphor and glass.
- Sadg4000 (talk) 22:47, 1 April 2014 (UTC)
- In a cold-cathode (glow discharge) lamp, the cathode life is exceptionally long. That's why they're used in LCD TVs and neon signs. While true that the high voltage causes excessive amounts of sputtering, most of the metal sputtered doesn't have the energy to escape the negative field, so it's redeposited right back onto the cathode, thus as long as your operating in the stable region then your lifespan is as high as it can go. (50,000 + hours are typical.) If talking about an arc lamp (excluding carbon arc), then simply leave it on all the time. It's switching them on and off that destroys the cathode, especially in the unstable region where it "sparks", or transitions to an arc. Zaereth (talk) 06:16, 13 December 2022 (UTC)
WikiProject class rating
[edit]This article was automatically assessed because at least one WikiProject had rated the article as start, and the rating on other projects was brought up to start class. BetacommandBot 09:49, 10 November 2007 (UTC)
Missing content
[edit]- cathode fall (also a main article is needed for this topic)
- generation of carriers
Rainald62 (talk) 09:30, 26 September 2010 (UTC)
- Voltage stabilisation (probably obsolescent, but once widely used)
- Negative resistance region (and simple neon oscillator)
Old Aylesburian (talk) 09:51, 3 December 2013 (UTC)
Arc vs glow
[edit]Professor Howatson says on pages 88-89 of a paperback I picked up years ago: " The arc is characterized by a higher current and a much lower voltage than the glow discharge; the arc voltage is typically less than 50 volts, an order of magnitude lower than the that of the glow discharge. The current density is much higher than in the glow discharge and the cathode region can no longer be distinguished, particularly since the high current density makes the positive column intensely luminous...It is possible to maintain an arc at low pressures (as, for example, in the mercury-arc rectifier) down to a few (microns); the characteristic high current density and low voltage drop are still found."
And all the other literature I've been reading seems to agree with him that arc discharges and glow discharges are usefully distinct phenomena. --Wtshymanski (talk) 01:17, 7 June 2011 (UTC)
- Whoops, I guess you're right. The book Plasma chemistry confirms it. Sorry about the confusion. I should've double-checked first. I guess I'll have to change the lede in the flashtube article to direct to the correct place. Thanks for catching that, Wtshymanski, and thanks to Achem as well. Zaereth (talk) 18:02, 7 June 2011 (UTC)
Sputtering discussion ill-placed and missing content
[edit]The whole paragraph on sputtering of the cathode is ill-placed in my view. The "basic operating mechanism" section should describe only the physics important for the discharge. Sputtering of the cathode, on the other hand, is not at all important for a glow discharge. It is merely a side effect. It should be discussed in a separate section. On the other hand some of the important physics is not discussed at all. Secondary electron emission at the cathode, which is important to create electrons that start at the cathode, is not mentioned at all. The mechanisms creating the various glows and dark spaces are also not really explained. — Preceding unsigned comment added by 130.183.16.154 (talk) 14:20, 22 June 2011 (UTC)
- I think you have some pretty good suggestions. I don't have time to work on this article right now, but someone may come along and read your suggestions, and decide to do something about it. However, if you think the article needs reorganizing, you are most welcome to go ahead and make the changes. If you are able to add some info to the article, then please go for it. Just remember to cite a reliable source and to rewrite the info in your own words. Thanks for your assistance and, if you have any questions, feel free to ask. Zaereth (talk) 21:27, 22 June 2011 (UTC)
- I agree with User:130.183.16.154; the introduction of sputtering makes the entire explanation of how a glow discharge works erroneous. In Basic operating mechanism (2nd para) it says:
- "The ions (which are positively charged) are driven towards the cathode by the electric potential... [when they] strike the cathode... a portion of the cathode is ejected, typically in the form of free atoms. This process is known as sputtering. Once free of the cathode, atoms move into the bulk of the glow discharge... The atoms can then be excited by collisions with ions, electrons, or other atoms...."
- This makes it sound like the atoms which are sputtered off the cathode are essential to a glow discharge; they are what give off the light. Although this mechanism may occur, I thought it was atoms of the gas, struck by electrons emitted by the cathode, that gave off the bulk of the light in a glow discharge. This section should be rewritten. --ChetvornoTALK 01:01, 18 July 2016 (UTC)
- I may have a little time to do some work on this in the near future. The thing about metal-vapor lamps is that the entire envelope needs to remain hotter than the vaporization point of the metal. The metal will be attracted to any cold spots where it will grow into a solid, amorphous coating (or liquefy in the case of mercury lamps). Because the melting point of tungsten is higher than any glass, you never see a tungsten-vapor lamp. The metal atoms that sputter don't make it very far from the cathode before they become stuck to the glass. Therefore, the light is produced almost solely by the gas.
- I agree with User:130.183.16.154; the introduction of sputtering makes the entire explanation of how a glow discharge works erroneous. In Basic operating mechanism (2nd para) it says:
- That said, sputter is always a big problem in low-energy applications like glow discharges. Because the cathode doesn't heat very much, is ejects fewer electrons, so more positive ions, with high kinetic energy and low thermal energy, end up coming into full contact (bombardment) with the cathode to get an electron, causing the sputter. Cathodes are often coated with a low work-function materiel to help better eject electrons, reducing sputter. Zaereth (talk) 23:59, 18 July 2016 (UTC)
- It seems to me that sputtering is a side effect in most applications of glow discharges: lamps, plasma TVs, etc. While it could be mentioned in Basic operating mechanism, I think a detailed discussion could be relegated to a separate section. Also, I think the arc in a high pressure metal-vapor lamp is an arc discharge, not a glow discharge? There should be a section describing the differences between the three types of electric discharge: Townsend discharge, glow discharge, and arc. --ChetvornoTALK 01:59, 19 July 2016 (UTC)
- I agree completely. Yes, metal-vapor lamps are arc lamps. I mentioned metal-vapor lamps only to demonstrate that glow-discharge lamps are incapable of using the sputtered atoms for any real light production, the envelope is too cold to sustain the vapor so it all just goes to the glass. Zaereth (talk) 02:42, 19 July 2016 (UTC)
- Ah, now I see. That's a good point. --ChetvornoTALK 23:50, 20 July 2016 (UTC)
- An interesting test is used on fluorescent lamps to measure the mercury loss over time, which simply involves placing a piece of ice (or gel pack for safety reasons) in contact with a small portion of the glass. It's amazing how quickly the light will dim as the mercury pools into a little bead. Doesn't matter what kind of lamp, mercury, glow-discharge, flashtube or even a gold-vapor laser, the electrode material almost always has a higher melting point than the envelope (even sapphire), thus is completely irrelevant to the light output. (An exception to this are liquid electrodes, like found in mercury-arc rectifiers, but for obvious reasons these are very inefficient sources of light.) Zaereth (talk) 01:16, 21 July 2016 (UTC)
- Awesome! That would be a wonderful demonstration for school science classes. I've got to try that with my desk lamp. --ChetvornoTALK 03:46, 21 July 2016 (UTC)
- An interesting test is used on fluorescent lamps to measure the mercury loss over time, which simply involves placing a piece of ice (or gel pack for safety reasons) in contact with a small portion of the glass. It's amazing how quickly the light will dim as the mercury pools into a little bead. Doesn't matter what kind of lamp, mercury, glow-discharge, flashtube or even a gold-vapor laser, the electrode material almost always has a higher melting point than the envelope (even sapphire), thus is completely irrelevant to the light output. (An exception to this are liquid electrodes, like found in mercury-arc rectifiers, but for obvious reasons these are very inefficient sources of light.) Zaereth (talk) 01:16, 21 July 2016 (UTC)
- I found the source for that, in the book Illuminating engineering -- Volume 63, on page 172. It says use dry ice for much better localized cooling of the glass. The pressure will drop rapidly (like a hole had been cut in the glass) as the mercury rushes to the cold spot. Something about metals; they really don't like being vapors. Zaereth (talk) 23:51, 22 July 2016 (UTC)
Color Of positive column with nature of gas
[edit]
In respect to Geissler's Discharge Rajnish45 (talk) 16:33, 14 July 2016 (UTC)
- I'm guessing you're referring to the difference in color between it and the negative zone. This is mainly due to pressure-differential and the different types of transitions that are occurring. In the positive column, emission is caused by low pressure, spectral lines of very narrow bandwidth. The highest concentrations of bound/bound collisions occur outside the so-called '"Faraday cones" while more free/bound transistions occur within. (You can picture the cones as a stack of magnets; negatively charged on the dark side while positively charged on the other, brighter side. The light spaces are filled with positive ions while dark-space in between is more of a vacuum filled with free electrons, stripped from those ions.) In the negative column, pressure is higher, energy density is higher, spectral lines are broader, and both free/bound and particularly free/free transitions produce more continuum radiation shifted toward the blue end of the spectrum. (There are far more electrons per ions, missing their them on their way to the anode and causing bremsstralung radiation.) Zaereth (talk) 08:22, 15 July 2016 (UTC)
- See figure at right. I don't see pressure being an influence because the pressure (and the mean free path) should be nearly constant in the tube. The fields are drastically different. High fields near the cathode allow higher energy collisions (hence higher energy/bluer transitions). Higher positive ion density allows more pumping. In the positive column, the field is low, so the typical electron doesn't have as much energy. +Ion density is lower. Glrx (talk) 18:54, 23 July 2016 (UTC)
- Well the charts refer to electrical flow, not necessarily to the dynamics occurring in the plasma itself. The plasma is electrically charged and doesn't necessarily behave as an ideal gas. The charged atoms instead follow the magnetic forces. For example, in an arc flash the plasma won't necessarily expand like an ideal gar, but will seek out any metallic surfaces, including things like ear-rings and belt buckles. The pressure within these plasma jets is higher than without, because of the mere concentration of ions. In an ablative flashtube, the magnetic forces compress nearly all of the plasma to the surface of the glass, so that the lamp performs like an axial lamp, reducing inductance and causing faster flashes. Pressure is always higher in the negative areas than in the positive, because that's where the ions are rushing to.
- The charts deal with the positive column as a single entity, and don't really explain the existence of the Faraday cones. However, the column is made up of three different particles (electrons, ions, and neutral atoms) interacting in different ways. First, consider a simple DC arc-lamp. Because it's DC, there is no skin effect so the current density is highest in the center of the arc. Thus, the highest concentration of continuum radiation comes from the center of the arc while more spectral lines occurs farther to the surface. Near the anode, neutral atoms lose their electrons before ever coming close to the anode itself. These neutral atoms ionize and speed away from the anode at incredible velocity, leaving only their free electrons on their way to the anode. Thus, a vacuum occurs between the anode and the glowing plasma. The free electrons are incapable of producing light, because only the ions do that, so a dark space near the anode results. There are few neutral atoms because they have all been ionized, thus a vacuum also results.
- In a glow discharge, the flow of electricity is not as free. The process that happens only near the anode in an arc lamp occurs several times in a glow-discharge. In the positive column, the central cones are like plasma "anodes' on one side, filled with both positive ions and neutral atoms. The highest concentration of ions are at the surface of the cones, where incoming free-electrons abound. As they capture the free electrons, the ions give off a photon. These atoms then are shoved back toward the anode by the incoming ions. About half way to the next cone. the neutral atoms are stripped of their electrons, so they speed toward the cathode while the free electrons speed to the anode, until the free electrons encounter the next Faraday cone, and the relay process happens all over again.
- In the meantime, current density and pressure are both lower outside the cones, so nearly the all of the light in this area is produced by collisions between ions and atoms. There are very few free electrons, so the light from this area is nearly all spectral lines. Zaereth (talk) 09:04, 25 July 2016 (UTC)
Copied from User talk:Chetvorno
[edit]There are several things to consider. It's actually the ions that are the most energetic particles in the plasma. The ions are most energetic near the cathode, while the electrons are most energetic when approaching the anode. The ions make up less than 1% of the plasma and produce all of the emitted light.
Electrons are far less energetic. The negative field around the cathode tends to always remain constant in size, whereas lengthening the tube will only cause the positive column to grow in length. By the time an electron reaches the end of the negative column, it's energy is almost depleted. It is at this point that most of the electrons move around the positive column, enclosing it inside an electron sheath. From this point, nearly all of the current flow occurs within this electron sheath, bypassing the gas. Contained by the sheath, the plasma in the positive column exchanges electrons neutrally (without taking in much from the cathode or putting much out to the anode), in the form of eddy currents.
There are three separate ways in which the plasma may produce light, and all three occur to some degree. When the ions and atoms collide, and electron is transferred from one to the other. This causes a photon to be emitted of one of a few very specific wavelengths (spectral lines). This method predominates at low current densities, and is responsible for the pinkish glow. At higher current densities, free-bound transitions tend to occur. This happens when an ion captures a free electron, and a photon of light of any wavelength will be emitted. Areas where the gas appears more whitish (in both the positive and negative column) is where these transitions are occurring. Free-free transitions happen when an electron is accelerated or decelerated by an ion, without being captured by it. This removes some energy from the ion, thus a photon of light is emitted at any wavelength. Free-free transitions increase with increasing energy density (where there is a high concentrations of ions per electrons), and this high energy causes the lower atomic levels to reach saturation very quickly, so the output tends to be centered in the blue to UV portions of the spectrum. Therefore, areas where the gas is blue or purplish is where these free-free transitions are occurring the most. The plasma is literally trying to tear itself apart in opposite directions. I hope that helps. Zaereth (talk) 23:31, 16 June 2017 (UTC)
Kinetic?
[edit]"... longer mean free path allows a charged particle to gain more energy before colliding with another particle ..."
should be?
"... gain more kinetic energy before colliding ..."
— Preceding unsigned comment added by 60.240.12.146 (talk • contribs) 05:52, 11 June 2019 (UTC)
Diagrams
[edit]
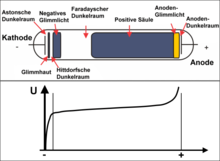
Good diagrams! In the top diagram, what is graph 1, labeled "I"? It can't be total current, as that is constant.
Below is a diagram I drew a while ago:

Why does glow surround the cathode?
[edit]In a neon bulb, one might expect the glow to exist only between the electrodes. On the outside of the electrodes, there should be no E-field, so why is there a glow? Can someone explain this? Thanks --185.254.136.122 (talk) 03:16, 13 December 2022 (UTC)
- This is going to be a little long winded, but plasma physics is a complicated thing. It's because at the cathode is where all the action happens. Keep in mind that the electricity flows in the opposite direction of power. At the anode, electrons are simply flooding into the metal, and since electrons produce no light on their own, there is not much to see there. The power is all in the ions, which accelerate in the opposite direction, toward the cathode, and it's the ions that produce all of the emitted light.
- The cathode is surrounded by a negative field. When the ions smash into the cathode, they eject electrons from the surface, which go flying up from the surface like meteorite ejecta. (This will also cause the heavier metals of the surface to be blown away and ejected as well.) The problem is, at such low temperatures (too low to form an arc), in addition to the voltage drop of the gas column, the area directly surrounding the cathode has a lot of resistance, which is called the cathode fall. Most of the electrons and metal atoms don't have enough energy to escape the negative field of the cathode, so they fall right back down and are redeposited right back onto its surface. As they are either being ejected or falling back, the electrons encounter passing ions, which are speeding to the cathode as fast as they can. These electrons tug on the ions as they pass, slowing them down. This produces Bremsstrahlung radiation (braking radiation), causing it to emit light in the deep blue to violet region of the spectrum.
- Very few electrons (and fewer metal atoms) actually have enough energy to make it out of the negative field. Those that do are basically exhausted of all their energy by the time they do, so they just kind of gather up in the Faraday dark space, like a slough in a river. Barely moving, they slowly begin to accelerate toward the anode, but with all that gas in the way, they instead decide it's much easier to just go around the positive column, so they split up and form an electron sheath around the gas. Nearly all of the electricity then flows in the dark spaces around the gas rather than through it, and thus produces no light.
- Believe it or not, nearly all of the light from the positive column is caused by eddy currents in the gas, produced via induction from the skin effect caused by the electron sheath. There is really no appreciable flow of electricity through the gas, like there is in an arc, but instead the electricity in the gas just goes around and around in little circles, which can be seen as the so-called "Faraday cones" in the gas.
- In nearly all lamps, the negative column is the same length, proportional to the diameter of the tube. The positive column, on the other hand, can extend to whatever length the tube is. Regions where the light is mostly blue is where Bremsstrahlung is happening. Areas where it appears more white is where ions are capturing free electrons, and areas where colored spectral-line radiation (pink in our photos) is occurring is where ions are colliding with neutral atoms to produce the light. I hope that helps explain. Zaereth (talk) 04:06, 13 December 2022 (UTC)
- Thank you @Zaereth for your explanation. But I don't understand one of your sentence : "Most of the electrons and metal atoms don't have enough energy to escape the negative field of the cathode, so they fall right back down and are redeposited right back onto its surface". The electric potential is the lowest at the cathode, so the electrons are always repelled by the cathode after they are emitted from it : how could they "fall right back down" to the cathode that repels them? Samuel.Damoy (talk) 20:43, 19 May 2023 (UTC)
- I don't know. That's what the books say. There hasn't been much research done on this since the 1930s, once Philips developed the basics of low work function cathodes that are still used in nearly all lamps today. Most of what people like Townsend and Faraday learned about discharges still holds water today.
- What I do know is, on an atomic level electrons repel each other, but as a larger group their fields combine to form an even larger field, and for some reason this larger field does not seem to repel them. That it's described as the field pulling them back is just a matter of convention; for example, we describe gravity as being a pull when the Theory of Relativity describes is as being more of a pushing force, as if when you throw a ball in the air it's actually the universe that pushes it back down. (Something similar to the way those last few Cheerios floating in your milk all clump together in groups from the milk's surface tension.) So why do electrons clump in groups? Is it simply the ion bombardment that they have to shoot through that pulls them back down or is there more to it?
- When I was 8 I rebuilt my first small engine, without any help or guidance, or even the proper tools. I figured out how the magneto worked, and that it could shock the living hell out of you, but I couldn't figure out why it worked. I mean, you pass a magnet by a coil it makes electricity; any kindergartener knows that. But no one could ever tell me just where all those electrons came from. They couldn't just appear out of thin air? Could they?
- It turns out that the answer to my question was quite simple, but took a genius to explain it to me simply. The electricity was always there. It was hiding inside the metal the whole time. In fact, it's all that electricity that gives a metal it's luster, ductility, and conductive properties. All the magnet does it move the electrons in a circle, like a pump moving water in a closed loop. Suddenly, the process of electrical generation made sense! But, like all knowledge, every answer only leads to more questions. If these electrons are repelling each other, then why don't they just fly right out of the metal? Something about our positive universe abhors a negative charge, so electrons are forced to hide away in metals. Why? I have yet to find out, but I'm sure once that question is answered it will lead to a hundred more questions, and the universe will become even bigger and more mysterious yet. Zaereth (talk) 01:28, 8 June 2023 (UTC)
- Thank you @Zaereth. I'm not sure all your examples are relevant here, but now I understand what you mean. I'm not an expert, but I have a master in material sciences, and a good experience with the subject (I'm ions sources engineer in nuclear physic's lab GANIL), so I may answer some of your questions :
- - The force which explains why "If these electrons are repelling each other, then why don't they just fly right out of the metal?" is due to the attraction from the positive nucleus in the metal. Of course this is oversimplified, and you are right about the importance of e- / e- repulsion in some cases, but when needed, some models provide good approximations for e- interactions, see for example Electronic band structure#Theory in crystals).
- - The small field that can make e- "fall right back down" can be understood as equivalent to image charges. This effect contributes to the work function you wisely mentioned.
- -At high emission current, Space charge effects can also decrease the field pulling the e- outside the cathode : this is due to previously emitted secondary e- moving away from the cathode, but still close enough to produce a small repulsive force toward the cathode, on the more recently emitted e-.
- I guess this is what you mean with "as a larger group their fields combine to form an even larger field, and for some reason this larger field does not seem to repel them", even if it's not very accurate because theses 2 effects are small here, and decrease exponentially : except at a very close proximity of the cathode's surface, theses effects are negligible, compared to the global electric field that accelerate the emitted e- away from the cathode, so here this is an unnecessary detail, not very important to explain glow discharge
- But anyway, I think all your contributions in the article are OK : my comment is just about your sentence in this talk (not in the article), I mentioned first, because it is not very relevant, and may be confusing, for the reasons that I hope you understand now. The important point is that the sentence in the article is OK, as it doesn't explain too many details about how e- escape from the cathode, which is sufficient to explain glow discharge : " Once free of the cathode, the electric field accelerates electrons into the bulk of the glow discharge". Samuel.Damoy (talk) 13:34, 11 June 2023 (UTC)
- It turns out that the answer to my question was quite simple, but took a genius to explain it to me simply. The electricity was always there. It was hiding inside the metal the whole time. In fact, it's all that electricity that gives a metal it's luster, ductility, and conductive properties. All the magnet does it move the electrons in a circle, like a pump moving water in a closed loop. Suddenly, the process of electrical generation made sense! But, like all knowledge, every answer only leads to more questions. If these electrons are repelling each other, then why don't they just fly right out of the metal? Something about our positive universe abhors a negative charge, so electrons are forced to hide away in metals. Why? I have yet to find out, but I'm sure once that question is answered it will lead to a hundred more questions, and the universe will become even bigger and more mysterious yet. Zaereth (talk) 01:28, 8 June 2023 (UTC)


