Potassium-40
 | |
| General | |
|---|---|
| Symbol | 40K |
| Names | potassium-40, 40K, K-40 |
| Protons (Z) | 19 |
| Neutrons (N) | 21 |
| Nuclide data | |
| Natural abundance | 0.0117(1)% |
| Half-life (t1/2) | 1.251(3)×109 y |
| Isotope mass | 39.96399848(21) Da |
| Spin | 4− |
| Excess energy | −33505 keV |
| Binding energy | 341523 keV |
| Parent isotopes | Primordial |
| Decay products | 40Ca (β−) 40Ar (EC, γ; β+) |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β− | 1.31109 |
| EC, γ | 1.5049 |
| Isotopes of potassium Complete table of nuclides | |
Potassium-40 (40K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 ppm) of natural potassium.
Potassium-40 undergoes four different types of radioactive decay, including all three main types of beta decay: electron emission (β−) to 40Ca with a decay energy of 1.31 MeV at 89.6% probability, positron emission (β+ to 40Ar at 0.001% probability[1], electron capture (EC) to 40Ar* followed by a gamma decay emitting a photon[Note 1] with an energy of 1.46 MeV at 10.3% probability and direct electron capture (EC) to the ground state of 40Ar at 0.1%.[2][3][4] Both forms of the electron capture decay release further photons[Note 2], when electrons from the outer shells fall into the inner shells to replace the electron taken from there.
The EC decay of 40K explains the large abundance of argon (nearly 1%) in the Earth's atmosphere, as well as prevalence of 40Ar over other isotopes.
Potassium–argon dating
[edit]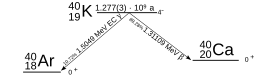
Potassium-40 is especially important in potassium–argon (K–Ar) dating. Argon is a gas that does not ordinarily combine with other elements. So, when a mineral forms – whether from molten rock, or from substances dissolved in water – it will be initially argon-free, even if there is some argon in the liquid. However, if the mineral contains traces of potassium, then decay of the 40K isotope present will create fresh argon-40 that will remain locked up in the mineral. Since the rate at which this conversion occurs is known, it is possible to determine the elapsed time since the mineral formed by measuring the ratio of 40K and 40Ar atoms contained in it.
The argon found in Earth's atmosphere is 99.6% 40Ar; whereas the argon in the Sun – and presumably in the primordial material that condensed into the planets – is mostly 36Ar, with less than 15% of 38Ar. It follows that most of Earth's argon derives from potassium-40 that decayed into argon-40, which eventually escaped to the atmosphere.
Contribution to natural radioactivity
[edit]
The decay of 40K in Earth's mantle ranks third, after 232Th and 238U, in the list of sources of radiogenic heat. Less is known about the amount of radiogenic sources in Earth's outer and inner core, which lie below the mantle. It has been proposed, though, that significant core radioactivity (1–2 TW) may be caused by high levels of U, Th and K.[5][6]
Potassium-40 is the largest source of natural radioactivity in animals including humans. A 70 kg human body contains about 140 g of potassium, hence about 140g × 0.0117% ≈ 16.4 mg of 40K;[7] whose decay produces about 3850[8] to 4300 disintegrations per second (becquerel) continuously throughout the life of an adult person (and proportionally less in young children).[Note 3][9]
Banana equivalent dose
[edit]Potassium-40 is famous for its usage in the banana equivalent dose, an informal unit of measure, primarily used in general educational settings, to compare radioactive dosages to the amount received by consuming one banana. The radioactive dosage from consuming one banana is around 10−7 sievert, or 0.1 microsievert, under the assumptions, that all of the radiation produced by potassium-40 is absorbed in the body (which is mostly true, as the majority of the radiation is beta-minus radiation, which has a short range) and that the biological half life of potassium-40 is around 30 days (which is likely too large an estimate, as the body controls potassium levels closely and emits excess potassium quickly through urine). At the estimated 0.1 µSv, one banana equivalent dose is around 1% of the average American's daily exposure to radiation.[10]
Other naturally occurring potassium isotopes
[edit]Besides the long lived potassium-40, there are also trace amounts of potassium-42 in the biosphere. Potassium-42 has a short half life of just over half a day, so exposure to it is usually through the air, but it cannot accumulate in longer lived plants or animals. Potassium-42 is produced by the natural decay of argon-42 with a half-life time of 32.9 years. Argon-42 is in turn produced mostly from nuclear reactions between highly energetic cosmic particles and atmospheric argon-40 in the outermost layers of the earth's atmosphere. Some argon-42 also originates from thermonuclear weapons testing, when the high neutron flux around these weapons lead to double neutron activation of atmospheric argon-40. Production rates are low though, with less than 1 in 1020 argon atoms being argon-42.[11]
See also
[edit]Notes
[edit]- ^ Also called a gamma ray, because it is produced by a transition in the nucleus
- ^ Also called x-ray, as they are emitted from transitions of electrons
- ^ The number of radioactive decays per second in a given mass of 40K is the number of atoms in that mass, divided by the average lifetime of a 40K atom in seconds. The number of atoms in one gram of 40K is the Avogadro constant 6.022×1023 mol−1 divided by the atomic weight of potassium-40 (39.96 g/mol): about 1.507×1022 per gram. As in any exponential decay, the average lifetime is the half-life divided by the natural logarithm of 2, or about 56.82×1015 seconds.
References
[edit]- ^ Engelkemeir, D. W.; Flynn, K. F.; Glendenin, L. E. (1962). "Positron Emission in the Decay of K40". Physical Review. 126 (5): 1818. Bibcode:1962PhRv..126.1818E. doi:10.1103/PhysRev.126.1818.
- ^ Stukel, M.; et al. (KDK Collaboration) (2024). "Rare 40K Decay with Implications for Fundamental Physics and Geochronology". Physical Review Letters. 131 (5): 052503. doi:10.1103/PhysRevLett.131.052503.
- ^ Hariasz, L.; et al. (KDK Collaboration) (2024). "Evidence for ground-state electron capture of 40K". Physical Review C. 108 (1): 014327. doi:10.1103/PhysRevC.108.014327.
- ^ "Physicists Observe Rare Nuclear Decay of Potassium Isotope". Sci.News. 2024-05-08. Retrieved 2024-05-08.
- ^ Wohlers, A.; Wood, B. J. (2015). "A Mercury-like component of early Earth yields uranium in the core and high mantle 142Nd". Nature. 520 (7547): 337–340. Bibcode:2015Natur.520..337W. doi:10.1038/nature14350. PMC 4413371. PMID 25877203.
- ^ Murthy, V. Rama; Van Westrenen, Wim; Fei, Yingwei (2003). "Experimental evidence that potassium is a substantial radioactive heat source in planetary cores". Nature. 423 (6936): 163–5. Bibcode:2003Natur.423..163M. doi:10.1038/nature01560. PMID 12736683. S2CID 4430068.
- ^ "Radioactive Human Body". Harvard Natural Sciences Lecture Demonstrations.
- ^ Connor, Nick. "What is Potassium-40 – Characteristics – Half-life – Definition". Radiation Dosimetry.
- ^ Bin Samat, S.; Green, S.; Beddoe, A. H. (1997). "The 40K activity of one gram of potassium". Physics in Medicine and Biology. 42 (2): 407–13. Bibcode:1997PMB....42..407S. doi:10.1088/0031-9155/42/2/012. PMID 9044422. S2CID 250778838.
- ^ Nick Connor (14 December 2019). "What is Banana Equivalent Dose – BED – Definition". Radiation Dosimetry.
- ^ Barabash, A.S.; Saakyan, R.R.; Umatov, V.I. (2016). "On concentration of 42Ar in the Earth's atmosphere". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. doi:10.1016/j.nima.2016.09.042.
External links
[edit]- Table of radioactive isotopes, K-40
- The Lund/LBNL Nuclear Data Search
- Potassium-40 Section, Radiological and Chemical Fact Sheets to Support Health Risk Analyses for Contaminated Areas
