Nickel(II) chloride
 Hexahydrate
| |
 Anhydrous
| |
| Names | |
|---|---|
| IUPAC name
Nickel(II) chloride
| |
| Other names
Nickelous chloride, nickel(II) salt of hydrochloric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.858 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3288 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NiCl2 | |
| Molar mass | 129.5994 g/mol (anhydrous) 237.69 g/mol (hexahydrate) |
| Appearance | yellow-brown crystals deliquescent (anhydrous) green crystals (hexahydrate) |
| Odor | odorless |
| Density | 3.55 g/cm3 (anhydrous) 1.92 g/cm3 (hexahydrate) |
| Melting point | 1,001 °C (1,834 °F; 1,274 K) (anhydrous) 140 °C (hexahydrate) |
| anhydrous 67.5 g/100 mL (25 °C) [1] 87.6 g/100 mL (100 °C) hexahydrate 282.5 g/100 mL (25 °C) [1] 578.5 g/100 mL (100 °C) | |
| Solubility | 0.8 g/100 mL (hydrazine) soluble in ethylene glycol, ethanol, ammonium hydroxide insoluble in ammonia, nitric acid |
| Acidity (pKa) | 4 (hexahydrate) |
| +6145.0·10−6 cm3/mol | |
| Structure | |
| Monoclinic | |
| octahedral at Ni | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
107 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−316 kJ·mol−1[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very toxic (T+) Irritant (Xi) Dangerous for the environment (N) Carcinogen |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H315, H317, H331, H334, H341, H350i, H360D, H372, H410 | |
| P201, P202, P260, P261, P264, P270, P271, P272, P273, P280, P281, P285, P301+P310, P302+P352, P304+P340, P304+P341, P308+P313, P311, P314, P321, P330, P332+P313, P333+P313, P342+P311, P362, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
105 mg/kg (rat, oral)[3] |
| Safety data sheet (SDS) | Fischer Scientific |
| Related compounds | |
Other anions
|
Nickel(II) fluoride Nickel(II) bromide Nickel(II) iodide |
Other cations
|
Palladium(II) chloride Platinum(II) chloride Platinum(II,IV) chloride Platinum(IV) chloride |
Related compounds
|
Cobalt(II) chloride Copper(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.[4]
Production and syntheses
[edit]Large scale production and uses of nickel chloride are associated with the purification of nickel from its ores. It is generated upon extraction nickel matte and residues obtained from roasting refining nickel-containing ores using hydrochloric acid. Electrolysis of nickel chloride solutions are used in the production of nickel metal. Other significant routes to nickel chloride arise from processing of ore concentrates such as various reactions involving copper chlorides:[5]
- NiS + 2 CuCl2 → NiCl2 + 2 CuCl + S
- NiO + 2 HCl → NiCl2 + H2O
Laboratory routes
[edit]Nickel chloride is not usually prepared in the laboratory because it is inexpensive and has a long shelf-life. The yellowish dihydrate, NiCl2·2H2O, is produced by heating the hexahydrate between 66 and 133 °C.[6] The hydrates convert to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas. Simply heating the hydrates does not afford the anhydrous dichloride.
- NiCl2·6H2O + 6 SOCl2 → NiCl2 + 6SO2 + 12HCl
The dehydration is accompanied by a color change from green to yellow.[7]
In case one needs a pure compound without presence of cobalt, nickel chloride can be obtained by cautiously heating hexaamminenickel chloride:[8]
Structure of NiCl2 and its hydrates
[edit]
NiCl2 adopts the CdCl2 structure.[9] In this motif, each Ni2+ center is coordinated to six Cl− centers, and each chloride is bonded to three Ni(II) centers. In NiCl2 the Ni-Cl bonds have "ionic character". Yellow NiBr2 and black NiI2 adopt similar structures, but with a different packing of the halides, adopting the CdI2 motif.
In contrast, NiCl2·6H2O consists of separated trans-[NiCl2(H2O)4] molecules linked more weakly to adjacent water molecules. Only four of the six water molecules in the formula is bound to the nickel, and the remaining two are water of crystallization, so the formula of nickel(II) chloride hexahydrate is [NiCl2(H2O)4]·2H2O.[9] Cobalt(II) chloride hexahydrate has a similar structure. The hexahydrate occurs in nature as the very rare mineral nickelbischofite.
The dihydrate NiCl2·2H2O adopts a structure intermediate between the hexahydrate and the anhydrous forms. It consists of infinite chains of NiCl2, wherein both chloride centers are bridging ligands. The trans sites on the octahedral centers occupied by aquo ligands.[10] A tetrahydrate NiCl2·4H2O is also known.
Reactions
[edit]Nickel(II) chloride solutions are acidic, with a pH of around 4 due to the hydrolysis of the Ni2+ ion.
Coordination complexes
[edit]
Most of the reactions ascribed to "nickel chloride" involve the hexahydrate, although specialized reactions require the anhydrous form.
Reactions starting from NiCl2·6H2O can be used to form a variety of nickel coordination complexes because the H2O ligands are rapidly displaced by ammonia, amines, thioethers, thiolates, and organophosphines. In some derivatives, the chloride remains within the coordination sphere, whereas chloride is displaced with highly basic ligands. Illustrative complexes include:
| Complex | Color | Magnetism | Geometry |
|---|---|---|---|
| [Ni(NH3)6]Cl2 | blue/violet | paramagnetic | octahedral |
| [Ni(en)3]2+ | violet | paramagnetic | octahedral |
| NiCl2(dppe) | orange | diamagnetic | square planar |
| [Ni(CN)4]2− | colorless | diamagnetic | square planar |
| [NiCl4]2− | blue[11] | paramagnetic[12] | tetrahedral[13] |
NiCl2 is the precursor to acetylacetonate complexes Ni(acac)2(H2O)2 and the benzene-soluble (Ni(acac)2)3, which is a precursor to Ni(1,5-cyclooctadiene)2, an important reagent in organonickel chemistry.
In the presence of water scavengers, hydrated nickel(II) chloride reacts with dimethoxyethane (dme) to form the molecular complex NiCl2(dme)2.[6] The dme ligands in this complex are labile.
Applications in organic synthesis
[edit]NiCl2 and its hydrate are occasionally useful in organic synthesis.[14]
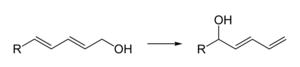
- As a mild Lewis acid, e.g. for the regioselective isomerization of dienols:
- In combination with CrCl2 for the coupling of an aldehyde and a vinylic iodide to give allylic alcohols.
- For selective reductions in the presence of LiAlH4, e.g. for the conversion of alkenes to alkanes.
- As a precursor to Brown's P-1 and P-2 nickel boride catalyst through reaction with NaBH4.
- As a precursor to finely divided Ni by reduction with Zn, for the reduction of aldehydes, alkenes, and nitro aromatic compounds. This reagent also promotes homo-coupling reactions, that is 2RX → R-R where R = aryl, vinyl.
- As a catalyst for making dialkyl arylphosphonates from phosphites and aryl iodide, ArI:
- ArI + P(OEt)3 → ArP(O)(OEt)2 + EtI
NiCl2-dme (or NiCl2-glyme) is used due to its increased solubility in comparison to the hexahydrate.[15]
Safety
[edit]Nickel(II) chloride is irritating upon ingestion, inhalation, skin contact, and eye contact. Prolonged inhalation exposure to nickel and its compounds has been linked to increased cancer risk to the lungs and nasal passages.[4]
References
[edit]- ^ a b Lide, David S. (2003). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. pp. 4–71. ISBN 9780849304842.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Nickel metal and other compounds (as Ni)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Grimsrud, Tom K; Andersen, Aage (2010). "Evidence of carcinogenicity in humans of water-soluble nickel salts". Journal of Occupational Medicine and Toxicology. 5 (1): 7. doi:10.1186/1745-6673-5-7. PMC 2868037. PMID 20377901.
- ^ Kerfoot, Derek G. E. (2000). "Nickel". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_157. ISBN 978-3-527-30385-4.
- ^ a b Ward, Laird G. L. (1972). "Anhydrous Nickel(II) Halides and their Tetrakis(ethanol) and 1,2-Dimethoxyethane Complexes". Inorganic Syntheses. Vol. 13. pp. 154–164. doi:10.1002/9780470132449.ch30. ISBN 9780470132449.
- ^ Pray, A. P. (1990). "Anhydrous Metal Chlorides". Inorganic Syntheses. Inorganic Syntheses. Vol. 28. pp. 321–2. doi:10.1002/9780470132593.ch80. ISBN 9780470132593.
- ^ Karyakin, Yu.V. (1947). Pure chemicals. Manual for laboratory preparation of inorganic substances (in Russian) (Moscow, Leningrad "State Scientific Technical Publishing of Chemical Literature" ed.). p. 416.
- ^ a b Wells, A. F. Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom, 1984.
- ^ B. Morosin "An X-ray diffraction study on nickel(II) chloride dihydrate" Acta Crystallogr. 1967. volume 23, pp. 630-634. doi:10.1107/S0365110X67003305
- ^ Gill, N. S. & Taylor, F. B. (1967). Tetrahalo Complexes of Dipositive Metals in the First Transition Series. Inorganic Syntheses. Vol. 9. pp. 136–142. doi:10.1002/9780470132401.ch37. ISBN 9780470132401.
- ^ Gerloch, M.; Slade, R. C. (1969). "Paramagnetic susceptibilities and anisotropies of nickel(II) ions in 'tetrahedral' environments. Part II. Covalency and distortion in tetraethylammonium tetrachloronickelate(II), and bis-(N-isopropyl-salicylaldiminato)nickel(II)". J. Chem. Soc. A. 0 (0): 1022–1028. doi:10.1039/J19690001022.
- ^ G. D. Stucky; J. B. Folkers; T. J. Kistenmacher (1967). "The Crystal and Molecular Structure of Tetraethylammonium Tetrachloronickelate(II)". Acta Crystallographica. 23 (6): 1064–1070. doi:10.1107/S0365110X67004268.
- ^ Tien-Yau Luh, Yu-Tsai Hsieh Nickel(II) Chloride" in Encyclopedia of Reagents for Organic Synthesis (L. A. Paquette, Ed.) 2001 J. Wiley & Sons, New York. doi:10.1002/047084289X.rn012. Article Online Posting Date: April 15, 2001.
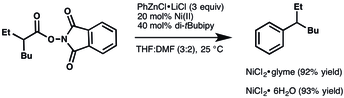
- ^ Cornella, Josep; Edwards, Jacob T.; Qin, Tian; Kawamura, Shuhei; Wang, Jie; Pan, Chung-Mao; Gianatassio, Ryan; Schmidt, Michael; Eastgate, Martin D. (2016-02-24). "Practical Ni-Catalyzed Aryl–Alkyl Cross-Coupling of Secondary Redox-Active Esters". Journal of the American Chemical Society. 138 (7): 2174–2177. doi:10.1021/jacs.6b00250. PMC 4768290. PMID 26835704.
External links
[edit]- NIOSH Pocket Guide to Chemical Hazards
- Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)


![{\displaystyle {\ce {{\overset {hexammine \atop nickel~chloride}{[Ni(NH3)6]Cl2}}->[175-200^{\circ }{\ce {C}}]NiCl2{}+6NH3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/196a5da2ce2ec8243534e7bf366995ac5327d0a4)