Milverine (drug)
 | |
| Clinical data | |
|---|---|
| Other names | Fenprin, Fenpyramine, Miospasm. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
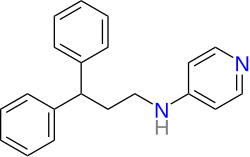
| Formula | C20H20N2 |
| Molar mass | 288.394 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Milverine is a spasmolytic (antispasmodic) agent that was developed in the latter half of the 20th century.[1][2][3]
The therapeutic use of fenpyramin as a platelet-antiaggregating and antithrombotic as well as vasodilating and antianginous medicine was also identified.[4]
- Milverine is a bifunctional molecule; 1 half of the molecule contains 3,3-Diphenylpropylamine and the other half of the molecule contains fampridine.
Synthesis
[edit]The chemical synthesis of milverine was identified.[5]
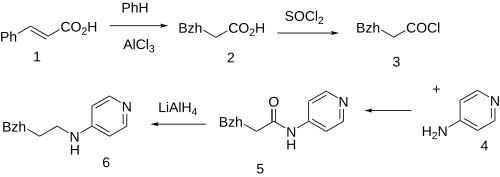
Conjugate soft addition of benzene to cinnamic acid [140-10-3] (1) gives 3,3-diphenylpropionic acid [606-83-7] (2). Halogenation with thionyl chloride gives 3,3-diphenylpropionyl chloride [37089-77-3] (3). Schotten-Baumann reaction with 4-aminopyridine (Fampridine) [504-24-5] (4) gives 3,3-diphenyl-N-(4-pyridyl)propionamide [75437-13-7] (5). The last step involves reduction of the amide bond giving milverine (6), respectively.
References
[edit]- ^ Carpenedo F, Santi-soncin E. [Spasmolytic activity of a new diphenylpropylaminic derivative, N-(4'-pyridyl)-3,3-diphenyl 1-aminopropane]. Minerva Med. 1970;61(43):2417-24. PMID: 5425728.
- ^ Baldi, F. et al, Curr. Ther. Res., 1984,36, 267 (pharmacol)
- ^ Fossati A, Sosio A, Pellegrini R, Frigo GM, D'Angelo L, Lecchini S, Marcoli M, Caravaggi M, Crema A. Milverine: in vitro and in vivo activity on the animal and human colon. Farmaco Prat. 1986 Sep;41(9):279-89. PMID: 3770157.
- ^ Rinaldo Pellegrini, Gaetano Clavenna, Piero Bellani, EP0143082 (1985 to RBS PHARMA (ROGER BELLON SCHOUM) S.p.A.).
- ^ Paolo Masi, Angela Monopoli, Adone Carlo Saravalle, Cesare Zio, DE3002909 (1980 to Italiana Schoum Spa).
