Main group peroxides
Appearance
It has been suggested that this article be merged with Metal peroxide to Inorganic peroxide. (Discuss) Proposed since November 2024. |
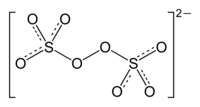
In chemistry, main group peroxides are peroxide derivatives of the main group elements. Many compounds of the main group elements form peroxides (R−O−O−R'), and a few are of commercial significance.[1]
Examples
[edit]With thousands of tons/year being produced annually, the peroxydisulfates, S2O2−8, are preeminent members of this class. These salts serve as initiators for polymerization of acrylates and styrene.[1]
At one time, sodium perborate was used in detergents. It has since largely been replaced by sodium carbonate sesquiperhydrate.[1]
Many peroxides are not commercially valuable but are of academic interest. One example is bis(trimethylsilyl) peroxide (Me3SiOOSiMe3).[2] Phosphorus oxides form a number of peroxides, e.g. "P2O6".[3]
References
[edit]- ^ a b c Jakob, Harald; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia; Gutewort, Sven (2007). "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
- ^ Jih Ru Hwu; Buh-Luen Chen; Santhosh F. Neelamkavil; Yuzhong Chen (2002). "Bis(trimethylsilyl) Peroxide". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rb219.pub3. ISBN 0-471-93623-5.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
