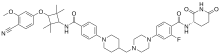
Luxdegalutamide
 | |
| Clinical data | |
|---|---|
| Other names | ARV-766 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C45H54FN7O6 |
| Molar mass | 807.968 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Luxdegalutamide, also known as ARV-766, is an investigational oral androgen receptor (AR) degrader being developed by Arvinas for the treatment of metastatic castration-resistant prostate cancer (mCRPC).[1][2] It belongs to a class of drugs called proteolysis targeting chimeras (PROTACs), which are designed to selectively degrade specific proteins by hijacking the ubiquitin-proteasome system.[3] Luxdegalutamide is a second-generation PROTAC AR degrader that has demonstrated a broader efficacy profile and better tolerability compared to its predecessor, ARV-110, in clinical settings.[3] It has shown promise in overcoming resistance associated with certain AR mutations, including the L702H mutation, which is prevalent in up to 24% of treated mCRPC patients.[3] As of 2024, luxdegalutamide is being evaluated in phase I/II clinical trials for prostate cancer.[1][2]
References
[edit]- ^ a b "Luxdegalutamide - Arvinas". AdisInsight. Springer Nature Switzerland AG.
- ^ a b Rej RK, Allu SR, Roy J, Acharyya RK, Kiran IN, Addepalli Y, et al. (April 2024). "Orally Bioavailable Proteolysis-Targeting Chimeras: An Innovative Approach in the Golden Era of Discovering Small-Molecule Cancer Drugs". Pharmaceuticals. 17 (4): 494. doi:10.3390/ph17040494. PMC 11054475. PMID 38675453.
- ^ a b c Israel JS, Marcelin LM, Thomas C, Szczyrbová E, Fuessel S, Puhr M, et al. (July 2024). "Emerging frontiers in androgen receptor research for prostate Cancer: insights from the 2nd international androgen receptor Symposium". Journal of Experimental & Clinical Cancer Research. 43 (1): 194. doi:10.1186/s13046-024-03125-5. PMC 11253403. PMID 39014480.
