List of miscellaneous 5HT2A agonists
This is a list of agonists of the serotonin receptor subtype 5-HT2A (and other 5-HT2 subtypes to a varying extent) which fall outside the common structural classes. Most agonists at this receptor are either substituted phenethylamine derivatives from the 2C, DOx and 25-NB groups, or substituted tryptamines and related compounds along with more complex derivatives of these such as lysergamides and iboga-type alkaloids.[1] There are however numerous 5-HT2A receptor agonists which do not fall within any of these groups, some representative examples of which are listed below. Ki and EC50 values vary depending on the assay conditions used and so may not be directly comparable between sources. Many of these compounds have been designed to be non-psychoactive derivatives for medical applications, and it should not be assumed that a compound which acts as a 5-HT2A agonist will necessarily be psychedelic in nature.[2]
| Structure | Name | Chemical name | h5-HT2A Ki (EC50) (nM) | PubChem | CAS number | Reference |
|---|---|---|---|---|---|---|

|
Compound 11a | 11-chloro-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,7-a]indole | 6.5 | 20726100 | 599173-28-1 | [3] |

|
Compound 23 | 9-Chloro-7-(2-ethoxy-phenyl)-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,7-a]indole | 32 | 44315398 | 599173-25-8 | [3] |

|
Compound 10d | 7-Benzyloxy-8-bromo-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine | 22 | 10472780 | 616201-60-6 | [4] |

|
Example 22.67 | 4-(6,7,8,9-tetrahydro-5H-pyrido[2,3-d]azepin-2-yl)thiomorpholine | 21 | 44124494 | [5] | |

|
Compound 3d (N-Bn-THAZ) | 2-benzyl-5,6,7,8-tetrahydro-4H-[1,2]oxazolo[4,5-d]azepin-3-one | (549) | 14515725 | 125115-66-4 | [6] |

|
Compound 11 | (3R)-N,N-diethyl-5-(1H-indol-4-yl)-1-methyl-3,6-dihydro-2H-pyridine-3-carboxamide | (<10) | 156278040 | [7] | |

|
Compound 106 | 6-chloro-1-(2,4,5-trimethoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole | 4376990 | 528525-37-3 | [8] | |

|
Compound 6c | (6S)-2,3-dichloro-7,8,9,10-tetrahydro-6H-6,9-epiminocyclohepta[b]quinoxaline | (400) | [9] | ||

|
Compound 70 | 3-(4-bromo-2-methylpyrazol-3-yl)-N-(4-chlorophenyl)-4-methoxyaniline | 1.77 | 9952456 | [10] | |

|
Compound 22 | 7-(trifluoromethoxy)-2,3,4,10b-tetrahydro-1H-pyrazino[1,2-b]isoindol-6-one | 67 (87) | 11448649 | [10] | |

|
Example 1 (ZC-B) | 3-(4-bromo-2,5-dimethoxyphenyl)azetidine | (1.6) | 156337249 | 2641630-65-9 | [11] |

|
2C-B-aminorex | 5-(4-bromo-2,5-dimethoxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 165360199 | [12] | ||

|
2-(2,5-dimethoxy-4-bromophenyl)morpholine | 2-(2,5-dimethoxy-4-bromophenyl)morpholine | 20.6 | 11429275 | [13] | |

|
DM-506 (ibogaminalog) | 3-methyl-2,4,5,6-tetrahydro-1H-azepino[4,5-b]indole | 24183 | 7546-66-9 | [14] | |

|
LPH-5 | (3S)-3-[2,5-dimethoxy-4-(trifluoromethyl)phenyl]piperidine | (3.2) | 156337168 | 2641630-97-7 | [15] |
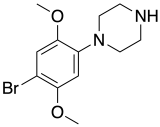
|
2C-B-PP | 1-(2,5-dimethoxy-4-bromophenyl)piperazine | 4738744 | 100939-87-5 | [16] | |

|
cis-urocanic acid (cis-UCA) | (Z)-3-(1H-imidazol-5-yl)prop-2-enoic acid | 4.6 | 1549103 | 7699-35-6 | [17][18][19] |

|
CPD-1 | (3S)-3-Methyl-1-[4-(trifluoromethyl)-1-benzofuran-7-yl]piperazine | 9925822 | 325145-37-7 | [20] | |

|
Efavirenz | (4S)-6-Chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one | 64139 | 154598-52-4 | [21] | |
| GM-2505 | [22] | |||||

|
IHCH-7079 | (6bR,10aS)-8-(2-Methoxyphenethyl)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline | 169488014 | 2957888-63-8 | [23] | |

|
IHCH-7113 | (6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline | 21302499 | 313368-85-3 | [23] | |
| ? | ITI-1549 | ? | 10.2 | ? | ? | [24] |

|
NDTDI | N,N-diethyl-3-[methyl(1,3,4,5-tetrahydrobenzo[cd]indol-4-yl)amino]propanamide | 163192742 | [25] | ||

|
Mefloquine | 2,8-bis(trifluoromethyl)quinolin-4-yl-(2-piperidyl)methanol | 40692 | 53230-10-7 | [26] | |

|
ORG-12962 | 1-(5-trifluoromethyl-6-chloropyridin-2-yl)piperazine | 9796408 | 210821-63-9 | [27] | |

|
ORG-37684 | (3S)-3-[(2,3-dihydro-5-methoxy-1H-inden-4-yl)oxy]pyrrolidine | 9794656 | 213007-95-5 | [28] | |

|
OSU-6162 | (3S)-3-[3-(methylsulfonyl)phenyl]-1-propylpiperidine | 9795741 | 156907-84-5 | [29] | |

|
PHA-57378 | 2,7,8,9,10,11-hexahydro-1H-azepino[4,5-b][1,4]oxazino[2,3,4-hi]indole | 4.1 | 10198481 | 303798-94-9 | [3] |

|
P-54 | 2-(5-methoxypyrazolo[1,5-a]pyridin-3-yl)-N,N-dimethylethanamine | 168946740 | [30] | ||
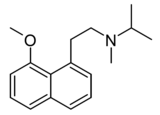
|
I-28 | N-[2-(8-methoxynaphthalen-1-yl)ethyl]-N-methylpropan-2-amine | 170604481 | [31] | ||

|
(R)-69 | 3-[(5R)-5-methyl-1,2,5,6-tetrahydropyridin-3-yl]-1H-pyrrolo[2,3-b]pyridine | 164513426 | [32] | ||

|
RS134-49 | 4-methyl-3-(1,2,3,6-tetrahydropyridin-5-yl)-1H-indole | 168941768 | [33][34] | ||

|
RH-34 | 3-[2-(2-methoxybenzylamino)ethyl]-1H-quinazoline-2,4-dione | 10041987 | 1028307-48-3 | [35] | |

|
SCHEMBL5334361 | 7-[(3-methoxyphenoxy)methyl]-2,3,4,5-tetrahydro-1H-3-benzazepine | (0.4) | 59027940 | 959867-47-1 | [36] |

|
Tabernanthalog | 8-methoxy-3-methyl-2,4,5,6-tetrahydro-1H-azepino[4,5-b]indole | 146026994 | 2483829-59-8 | [37] | |

|
TKU-II-100 | [(1S,2S)-2-(2-fluorophenyl)cyclopropyl]methanamine | 0.62 | 44572747 | [38] | |

|
WAY-470 | 1,16-diazatetracyclo[8.8.1.02,9.014,19]nonadeca-2(9),10,12,14(19)-tetraene | 36 | 10037962 | [39] | |

|
WXVL_BT0793LQ2118 | 6-fluoro-4-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-1H-indole | [40] | |||

|
Z2825713589 | (4-amino-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrol-2-yl)-(6-methoxypyrazin-2-yl)methanone | 167788805 | [40] | ||

|
Z2876442907 | ethyl 2-[[2-(4-methyl-1H-indol-3-yl)ethylamino]methyl]-1,3-thiazole-5-carboxylate | 167850865 | [40] | ||

|
Z3517967757 | 4-[1-(1-pyrimidin-2-ylethyl)piperidin-3-yl]phenol | 167949972 | [40] | ||

|
Z3881312504 | 2-bromo-4-[2-[methyl-[2-(1,3-thiazol-2-yl)ethyl]amino]ethyl]phenol | 167904469 | [40] | ||

|
Z4154032166 | 2,2,2-trifluoro-1-[6-(1,2,3,6-tetrahydropyridin-5-yl)pyridin-2-yl]ethanol | 167878716 | [40] | ||

|
Z5247692566 | 4-[(3,3-dimethyloxolan-2-yl)methyl]-3-[(1H-indol-3-yl)methyl]morpholine | [40] | |||

|
Z5247692629 | 1-(1-bicyclo[1.1.1]pentanyl)-4-[[5-(4-chlorophenyl)-1H-pyrazol-4-yl]methyl]piperazine | 166358273 | [40] |
See also
[edit]- DPCPX
- LY-341,495
- Robalzotan
- WAY-100635
- UH-301
- Head-twitch response
- Substituted benzofuran
- Substituted phenylmorpholine
- List of fentanyl analogues
References
[edit]- ^ Nichols DE (2018). "Chemistry and Structure-Activity Relationships of Psychedelics". Current Topics in Behavioral Neurosciences. 36: 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
- ^ Duan W, Cao D, Wang S, Cheng J (January 2024). "Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants". Chemical Reviews. 124 (1): 124–163. doi:10.1021/acs.chemrev.3c00375. PMID 38033123. S2CID 265512698.
- ^ a b c Ennis MD, Hoffman RL, Ghazal NB, Olson RM, Knauer CS, Chio CL, et al. (July 2003). "2,3,4,5-tetrahydro- and 2,3,4,5,11,11a-hexahydro-1H-[1,4]diazepino[1,7-a]indoles: new templates for 5-HT(2C) agonists". Bioorganic & Medicinal Chemistry Letters. 13 (14): 2369–2372. doi:10.1016/s0960-894x(03)00403-7. PMID 12824036.
- ^ Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, et al. (March 2005). "Discovery and SAR of new benzazepines as potent and selective 5-HT(2C) receptor agonists for the treatment of obesity". Bioorganic & Medicinal Chemistry Letters. 15 (5): 1467–1470. doi:10.1016/j.bmcl.2004.12.080. PMID 15713408.
- ^ WO 2009/079765, Slassi A, Isaac M, Xin T, Higgins G, He Z, Sun G, Quach T, "Compounds with activity at the 5-ht2c receptor", published 2 July 2009, assigned to Cascade Therapeutics Inc.
- ^ Jensen AA, Plath N, Pedersen MH, Isberg V, Krall J, Wellendorph P, et al. (February 2013). "Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties". Journal of Medicinal Chemistry. 56 (3): 1211–1227. doi:10.1021/jm301656h. PMID 23301527.
- ^ WO 2021/076572, Olson DE, Dunlap L, Wagner F, Chytil M, Powell NA, "Ergoline-like compounds for promoting neural plasticity", published 22 April 2021, assigned to The Regents Of The University Of California and Delix Therapeutics, Inc.
- ^ Orr MJ, Cao AB, Wang CT, Gaisin A, Csakai A, Friswold AP, et al. (April 2022). "Discovery of Highly Potent Serotonin 5-HT2 Receptor Agonists Inspired by Heteroyohimbine Natural Products". ACS Medicinal Chemistry Letters. 13 (4): 648–657. doi:10.1021/acsmedchemlett.1c00694. PMC 9014500. PMID 35450369.
- ^ Yao R, Jensen AA, Bryce-Rogers HP, Schultz-Knudsen K, Zhou L, Hovendal NP, et al. (August 2023). "Identification of 5-HT2 Serotonin Receptor Modulators through the Synthesis of a Diverse, Tropane- and Quinuclidine-alkaloid-Inspired Compound Library". Journal of Medicinal Chemistry. 66 (16): 11536–11554. doi:10.1021/acs.jmedchem.3c01059. PMID 37566000. S2CID 260806387.
- ^ a b WO 2004/058722, Teegarden B, Jayakumar H, Strah-Pleynet S, "Diarylamine and arylheteroarylamine pyrazole derivatives as modulators of 5ht2a.", published 15 July 2004, assigned to Arena Pharmaceuticals, Inc.
- ^ US 2021/0137908, Kristensen JL, Jensen AA, Märcher-Rørsted E, "5-HT2A Agonists for Use in Treatment of Depression.", published 13 May 2021, assigned to Lophora ApS.
- ^ Evans-Brown M, Gallegos A, Christie R, Jorge R, De Morais J, Almeida A, et al. (European Monitoring Center for Drugs and Drug Addiction) (December 2020). New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic. An update from the EU Early Warning System (PDF). Luxembourg: Publications Office of the European Union. doi:10.2810/921262. ISBN 978-92-9497-557-7.
- ^ Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, et al. (November 2004). "Beta-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 47 (24): 6034–41. doi:10.1021/jm040082s. PMID 15537358.
- ^ Arias HR, Rudin D, Hines DJ, Contreras A, Gulsevin A, Manetti D, et al. (March 2024). "The novel non-hallucinogenic compound DM506 (3-methyl-1,2,3,4,5,6-hexahydroazepino[4,5-b]indole) induces sedative- and anxiolytic-like activity in mice by a mechanism involving 5-HT2A receptor activation". European Journal of Pharmacology. 966: 176329. doi:10.1016/j.ejphar.2024.176329. hdl:2158/1354752. PMID 38253116.
- ^ Rorsted EM, Jensen AA, Smits G, Frydenvang K, Kristensen JL (May 2024). "Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists". Journal of Medicinal Chemistry. 67 (9): 7224–7244. doi:10.1021/acs.jmedchem.4c00082. PMC 11089506. PMID 38648420.
- ^ Lyon RA, Titeler M, McKenney JD, Magee PS, Glennon RA (May 1986). "Synthesis and evaluation of phenyl- and benzoylpiperazines as potential serotonergic agents". Journal of Medicinal Chemistry. 29 (5): 630–634. doi:10.1021/jm00155a008. PMID 3701781.
- ^ Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, et al. (November 2006). "Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor". Proceedings of the National Academy of Sciences of the United States of America. 103 (46): 17420–17425. doi:10.1073/pnas.0603119103. PMC 1859944. PMID 17085585.
- ^ Shen L, Ji HF (September 2009). "Molecular basis for cis-urocanic acid as a 5-HT(2A) receptor agonist". Bioorganic & Medicinal Chemistry Letters. 19 (18): 5307–5309. doi:10.1016/j.bmcl.2009.07.143. PMID 19683920.
- ^ Menezes AC, Raposo S, Simões S, Ribeiro H, Oliveira H, Ascenso A (March 2016). "Prevention of Photocarcinogenesis by Agonists of 5-HT1A and Antagonists of 5-HT2A Receptors". Molecular Neurobiology. 53 (2): 1145–1164. doi:10.1007/s12035-014-9068-z. PMID 25589005. S2CID 14282500.
- ^ Ahmed A, Choo H, Cho YS, Park WK, Pae AN (July 2009). "Identification of novel serotonin 2C receptor ligands by sequential virtual screening". Bioorganic & Medicinal Chemistry. 17 (13): 4559–4568. doi:10.1016/j.bmc.2009.05.003. PMID 19464901.
- ^ Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, González-Maeso J, et al. (November 2013). "The HIV antiretroviral drug efavirenz has LSD-like properties". Neuropsychopharmacology. 38 (12): 2373–2384. doi:10.1038/npp.2013.135. PMC 3799056. PMID 23702798.
- ^ Conference-poster: Hughes Z, Klein A, Dvorak D, Austin E, Kiss L, Marek G, et al. (2023). "22. GM-2505 has Rapid Onset Antidepressant Activity and Causes Dose-Dependent Changes in qEEG With Increasing 5-HT2A Receptor Occupancy". Biological Psychiatry. 93 (9): S102–S103. doi:10.1016/j.biopsych.2023.02.262.
- ^ a b Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. (January 2022). "Structure-based discovery of nonhallucinogenic psychedelic analogs". Science. 375 (6579): 403–411. Bibcode:2022Sci...375..403C. doi:10.1126/science.abl8615. PMID 35084960. S2CID 246360313.
- ^ Davis R, Dutheil SS, Zhang L, Lehmann E, Awadallah N, Yao W, et al. (December 2023). "ACNP 62nd Annual Meeting: Poster Abstracts P251 - P500: P358. Discovery and Characterization of ITI-1549, a Novel Non-Hallucinogenic Psychedelic for the Treatment of Neuropsychiatric Disorders". Neuropsychopharmacology. 48 (Suppl 1): 211–354 (272–273). doi:10.1038/s41386-023-01756-4. PMC 10729596. PMID 38040810.
- ^ "Analytical Report NDTDI" (PDF). Nacionalni Forenzični Laboratorij. Slovenia. June 2017.
- ^ Janowsky A, Eshleman AJ, Johnson RA, Wolfrum KM, Hinrichs DJ, Yang J, et al. (July 2014). "Mefloquine and psychotomimetics share neurotransmitter receptor and transporter interactions in vitro". Psychopharmacology. 231 (14): 2771–2783. doi:10.1007/s00213-014-3446-0. PMC 4097020. PMID 24488404.
- ^ Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. (September 1999). "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". British Journal of Pharmacology. 128 (1): 13–20. doi:10.1038/sj.bjp.0702751. PMC 1571597. PMID 10498829.
- ^ Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, et al. (August 2004). "Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 370 (2): 114–123. doi:10.1007/s00210-004-0951-4. PMID 15322733. S2CID 8938111.
- ^ Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, et al. (November 2011). "I. In vivo evidence for partial agonist effects of (-)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors". Journal of Neural Transmission. 118 (11): 1511–1522. doi:10.1007/s00702-011-0704-8. PMID 21874578. S2CID 43580367.
- ^ WO 2023115165, Banister S, Jorgensen W, Jinlong T, "Compounds", published 29 June 2023, assigned to Psylo Pty Ltd.
- ^ WO 2023230649, Banister S, Jorgensen W, Jinlong T, Whish L, "Compounds", published 7 December 2023, assigned to Psylo Pty Ltd.
- ^ Kaplan AL, Confair DN, Kim K, Barros-Álvarez X, Rodriguiz RM, Yang Y, et al. (October 2022). "Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity". Nature. 610 (7932): 582–591. Bibcode:2022Natur.610..582K. doi:10.1038/s41586-022-05258-z. PMC 9996387. PMID 36171289. S2CID 252598838.
- ^ Kargbo RB (November 2023). "Small-Molecule Heterocyclic Compounds: Gq-Biased Agonists for the 5-HT2A Receptor in Neuropsychiatric Treatment". ACS Medicinal Chemistry Letters. 14 (11): 1498–1500. doi:10.1021/acsmedchemlett.3c00444. PMC 10641896. PMID 37974947.
- ^ WO 2023114472, Manish J, Slocum S, Skiniotis G, Barros X, Jin J, Kaniskan HU, Sun N, Xiong Y, Shen Y, Xu Z, Qui X, Qian C, Song X, Deng Z, Roth B, Biberto J, Kuglae K, Suomivuori CM, Daemgen MA, Dror R, Shoichet B, Kaplan AL, "Heterocyclic compounds as 5ht2a biased agonists.", published 22 June 2023, assigned to Icahn School of Medicine at Mount Sinai, The University of North Carolina at Chapel Hill, The Board of Trustees of The Leland Stanford Junior University, The Regents of The University of California
- ^ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- ^ WO 2007149728, Mohapatra S, Hellberg MR, Feng Z, "Aryl and heteroaryl tetrahydrobenzazepine derivatives and their use for treating glaucoma", assigned to Alcon Manufacturing, Ltd.
- ^ Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- ^ WO 2007/025144, Kozikowski A, Kurome T, Setola V, Roth B, "5-ht2c receptor agonists as anorectic agents", published 1 March 2007, assigned to University Of Illinois - Chicago
- ^ Sabb AL, Vogel RL, Welmaker GS, Sabalski JE, Coupet J, Dunlop J, et al. (May 2004). "Cycloalkyl[b][1,4]benzodiazepinoindoles are agonists at the human 5-HT2C receptor". Bioorganic & Medicinal Chemistry Letters. 14 (10): 2603–7. doi:10.1016/j.bmcl.2004.02.100. PMID 15109661.
- ^ a b c d e f g h Lyu J, Kapolka N, Gumpper R, Alon A, Wang L, Jain MK, et al. (December 2023). "AlphaFold2 structures template ligand discovery". bioRxiv. doi:10.1101/2023.12.20.572662. PMC 10769324. PMID 38187536.
