Kynuramine
Appearance
| |
| Names | |
|---|---|
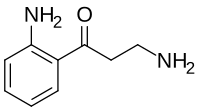
| IUPAC name
3-Amino-1-(2-aminophenyl)propan-1-one
| |
| Other names
Diaminopropiophenone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H12N2O | |
| Molar mass | 164.208 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Kynuramine is a chemical compound with the molecular formula C9H12N2O. It is the prototypical member of the class of biogenic amines known collectively as kynuramines.[1] Kynuramine is produced by the decarboxylation of kynurenine[1] and is a metabolite of tryptophan.[2]
Kynuramine is an α-adrenoceptor inhibitor.[3]
In biochemistry, kynuramine has been used as a substrate in assays used to measure amine oxidase activity.[4][5]
References
[edit]- ^ a b Hardeland, Rüdiger; Tan, Dun-Xian; Reiter, Russel J. (2009). "Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines". Journal of Pineal Research. 47 (2): 109–126. doi:10.1111/j.1600-079X.2009.00701.x. PMID 19573038.
- ^ Ruddick, Jon P.; Evans, Andrew K.; Nutt, David J.; Lightman, Stafford L.; Rook, Graham A.W.; Lowry, Christopher A. (2006). "Tryptophan metabolism in the central nervous system: Medical implications". Expert Reviews in Molecular Medicine. 8 (20): 1–27. doi:10.1017/S1462399406000068. PMID 16942634.
- ^ Johnson, Thomas D.; Clarke, David E. (1981). "An α-adrenoceptor inhibitory action of kynuramine". European Journal of Pharmacology. 72 (4): 351–356. doi:10.1016/0014-2999(81)90574-4. PMID 6115758.
- ^ Massey, J.B.; Churchich, J.E. (1977). "Kynuramine, a fluorescent substrate and probe of plasma amine oxidase". Journal of Biological Chemistry. 252 (22): 8081–8084. doi:10.1016/S0021-9258(17)40939-2. PMID 562342.
- ^ Matsumoto, T.; Suzuki, O.; Furuta, T.; Asai, M.; Kurokawa, Y.; Nimura, Y.; Katsumata, Y.; Takahashi, I. (1985). "A sensitive fluorometric assay for serum monoamine oxidase with kynuramine as substrate". Clinical Biochemistry. 18 (2): 126–129. doi:10.1016/S0009-9120(85)80094-1. PMID 4017223.
