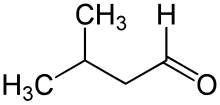
Isovaleraldehyde
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutanal | |
| Other names
Isovaleral, Isovaleric Aldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.811 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.13[1] |
| Appearance | Colorless Liquid[1] |
| Density | 0.785 g/mL at 20 °C[1] |
| Melting point | −51 °C (−60 °F; 222 K)[1] |
| Boiling point | 92 °C (198 °F; 365 K)[1] |
| Soluble in alcohol and ether, slightly soluble in water[1] | |
| −57.5×10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Combustible[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isovaleraldehyde organic compound, also known as 3-methylbutanal, with the formula (CH3)2CHCH2CHO. It is an aldehyde, a colorless liquid at STP,[1] and found in low concentrations in many types of food.[2] Commercially it is used as a reagent for the production of pharmaceuticals, perfumes and pesticides.[3]
Synthesis
[edit]Synthetic routes for the production of isovaleraldehyde vary. One method is by the hydroformylation of isobutene:
- (CH3)2C=CH2 + H2 + CO → (CH3)2C−CH2CHO
A small amount of 2,2-dimethylpropanal ((CH3)2C−C(CHO)CH3 side product is also generated.[3]
Another method of production involves the isomerization of 3-methylbut-3-en-1-ol using CuO–ZnO as a catalyst. A mixture of 3-methylbut-3-en-1-ol and 3-methylbut-2-en-1-ol may also be used. These starting materials are obtained from a reaction between isobutene and formaldehyde:[3]
- CH3CH3CCH2 + CH2O → (CH3)2CHCH2CHO
Finally, in beer the compound is produced via a reaction between the amino acid leucine and reductones in the malt.[4]
Occurrences and uses
[edit]As it can be derived from leucine, the occurrence of isovaleraldehyde is not limited to beer. The compound has found to be a flavor component in many different types of foods. It is described as having a malty flavor and has been found in cheese, coffee, chicken, fish, chocolate, olive oil, and tea.[2][5]
The compound is used as a reactant in the synthesis of a number of compounds. Notably it is used to synthesize 2,3-dimethylbut-2-ene, and is then converted to 2,3-dimethylbutane-2,3-diol and methyl tert-butyl ketone, better known as pinacolone. Pinacolone itself is then used in synthesis for number of pesticides. Additionally, a range of pharmaceuticals, such as butizide, are synthesized from isovaleraldehyde and its corresponding acid.[3] It is a common reagent or building block in organic synthesis.[6][7]

Acid-catalyzed cyclic trimerization of Isovaleraldehyde gives 2,4,6-Triisobutyl-1,3,5-trioxane [68165-40-2]. This is a flavouring agent that can be used in confectionary, tobacco, and other foodstuffs, toothpastes and the like.[8] It is described as imparting a creamy, dairy, vanilla chocolate and berry flavour.
According to IFF, isovaleraldehyde is used as a food flavorant additive.[9]
References
[edit]- ^ a b c d e f g h Lewis, R. J. Sr., ed. (2007). Hawley's Condensed Chemical Dictionary (15th ed.). New York, NY: John Wiley. p. 719.
- ^ a b Cserháti, T.; Forgács, E. (2003). "Flavor (Flavour) Compounds: Structures and Characteristics". Encyclopedia of Food Sciences and Nutrition (2nd ed.). Elsevier Science. pp. 2509–2517.
- ^ a b c d Kohlpaintner, C. "Aliphatic Aldehydes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. p. 9. doi:10.1002/14356007.a01_321.pub3. ISBN 978-3527306732.
- ^ Bamforth, C. W. (2003). "Chemistry of Brewing". Encyclopedia of Food Sciences and Nutrition (2nd ed.). pp. 440–447.
- ^ Owuor, P. O. (2003). "Tea: Analysis and Tasting". Encyclopedia of Food Sciences and Nutrition (2nd ed.). pp. 5757–5762.
- ^ Boeckman, Robert; Tusch, Douglas J.; Biegasiewiczjournal=Organic Syntheses, Kyle F. (2015). "Organocatalyzed Direct Asymmetric α-Hydroxymethylation of Aldehydes". Organic Syntheses. 92: 320–327. doi:10.15227/orgsyn.092.0320.
- ^ Poulsen, Pernille; Overgaard, Mette; Jensen, Kim L.; Jørgensenjournal=Organic Syntheses, Karl Anker (2014). "Enantioselective Organocatalytic α-Arylation of Aldehydes". Organic Syntheses. 91: 175–184. doi:10.15227/orgsyn.091.0175.
- ^ Donald Arthur Withycombe, et al. U.S. patent 4,093,752, U.S. patent 4,191,785 (1978 to International Flavors and Fragrances Inc).
- ^ "LMR Naturals".
