From Wikipedia, the free encyclopedia
Glyceollin III
Names
Preferred IUPAC name
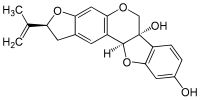
(2S ,6aS ,11aS )-2-(Prop-1-en-2-yl)-1,2-dihydro-6H -[1]benzofuro[3,2-c ]furo[3,2-g ][1]benzopyran-6a,9(11aH )-diol
Identifiers
ChEBI
ChEMBL
ChemSpider
KEGG
UNII
InChI=1S/C20H18O5/c1-10(2)15-6-11-5-13-17(8-16(11)24-15)23-9-20(22)14-4-3-12(21)7-18(14)25-19(13)20/h3-5,7-8,15,19,21-22H,1,6,9H2,2H3/t15-,19-,20+/m0/s1
Key: MIYTVBARXCVVHZ-RYGJVYDSSA-N
CC(=C)[C@@H]1Cc2cc3c(cc2O1)OC[C@@]4([C@H]3Oc5c4ccc(c5)O)O
Properties
C20 H18 O5
Molar mass
338.35 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Glyceollin III is a glyceollin , a type of pterocarpan , found in the soybean (Glycine max [ 1] [ 2] antiestrogenic effect.[ 3] antifungal activity against Aspergillus sojae [ 4]
^ Zimmermann, M. Carla; Tilghman, Syreeta L.; Boué, Stephen M.; Salvo, Virgilio A.; Elliott, Steven; Williams, K. Y.; Skripnikova, Elena V.; Ashe, Hasina; Payton-Stewart, Florastina; Vanhoy-Rhodes, Lyndsay; Fonseca, Juan Pablo; Corbitt, Cynthia; Collins-Burow, Bridgette M.; Howell, Melanie H.; Lacey, Michelle; Shih, Betty Y.; Carter-Wientjes, Carol; Cleveland, Thomas E.; McLachlan, John A.; Wiese, Thomas E.; Beckman, Barbara S.; Burow, Matthew E. (2010). "Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy" . Journal of Pharmacology and Experimental Therapeutics . 332 (1): 35– 45. doi :10.1124/jpet.109.160382 . PMC 2802480 PMID 19797619 . ^ Banks, Stephen W.; Dewick, Paul M. (1983). "Biosynthesis of glyceollins I, II and III in soybean". Phytochemistry . 22 (12): 2729– 2733. doi :10.1016/S0031-9422(00)97682-9 . ^ Salvo Virgilo A., Boue Stephen M., Fonseca Juan P., Elliott Steven, Corbitt Cynthia, Collins-Burow Bridgette M., Curiel Tyler J., Srivastav Sudesh K., Shih Betty Y., Carter-Wientjes Carol, Wood Charles E., Erhardt Paulw., Beckman Barbara S., McLachlan John A., Cleveland Thomas E. and Burow Matthew E. (2006). "Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis" . Clinical Cancer Research . 12 (23): 7159– 7164. doi :10.1158/1078-0432.CCR-06-1426 . PMID 17145841 . {{cite journal }}: CS1 maint: multiple names: authors list (link )^ Kim, Hyo Jung; Suh, Hwa-Jin; Lee, Choong Hwan; Kim, Jeong Hwan; Kang, Sun Chul; Park, Sunmin; Kim, Jong-Sang (2010). "Antifungal Activity of Glyceollins Isolated from Soybean Elicited with Aspergillus sojae". Journal of Agricultural and Food Chemistry . 58 (17): 9483– 9487. doi :10.1021/jf101694t . PMID 20666365 .
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown