Draft:Spiclomazine
 | Draft article not currently submitted for review.
This is a draft Articles for creation (AfC) submission. It is not currently pending review. While there are no deadlines, abandoned drafts may be deleted after six months. To edit the draft click on the "Edit" tab at the top of the window. To be accepted, a draft should:
It is strongly discouraged to write about yourself, your business or employer. If you do so, you must declare it. Where to get help
How to improve a draft
You can also browse Wikipedia:Featured articles and Wikipedia:Good articles to find examples of Wikipedia's best writing on topics similar to your proposed article. Improving your odds of a speedy review To improve your odds of a faster review, tag your draft with relevant WikiProject tags using the button below. This will let reviewers know a new draft has been submitted in their area of interest. For instance, if you wrote about a female astronomer, you would want to add the Biography, Astronomy, and Women scientists tags. Editor resources
Last edited by Citation bot (talk | contribs) 7 minutes ago. (Update) |
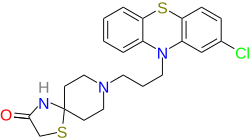 | |
| Clinical data | |
|---|---|
| Other names | Clospirazine, APY-606, Disepron. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H24ClN3OS2 |
| Molar mass | 446.02 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
 | |
| Clinical data | |
|---|---|
| Other names | Clospirazine, APY-606, Disepron. |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H24ClN3OS2 |
| Molar mass | 446.02 g·mol−1 |
| 3D model (JSmol) | |
| |
Neuroleptic Tranquilliser (used in treatment of schizophrenia).
Pharmacol:[1][2] Antineoplastic:[3]
Synthesis
[edit]A chemical synthesis is proposed:[4][5][6]

The reaction between the hydrochloride of 4-piperidone [41661-47-6] HCl H2O [40064-34-4] (1), ammonium carbonate [506-87-6] (2) and thioglycolic acid [68-11-1] (3) gives 1-thia-4,8-diazaspiro[4.5]decan-3-one, PC10419642 (4). The alkylation of the last with 2-chloro-10-(3-chloropropyl)phenothiazine [2765-59-5] (5) completed the synthesis of Spiclomazine (6)
References
[edit]- ^ Nakanishi M, Okada T, Tsumagari T (July 1970). "[Studies on psychotropic drugs. V. Pharmacological effects of 8-(3-(2-chloro-10-phenothiazinyl)propyl)-1-thia-4,8-diazaspiro(4,5) decan-3-one hydrochloride (APY-606)]". Yakugaku Zasshi : Journal of the Pharmaceutical Society of Japan (in Japanese). 90 (7): 800–7. doi:10.1248/yakushi1947.90.7_800. PMID 5465390.
- ^ Nakanishi M, Okada T, Kato Y (July 1970). "[Studies on psychotropic drugs. VI. Metabolic fate of APY-606. (1). Excretion and metabolism in rats]". Yakugaku Zasshi : Journal of the Pharmaceutical Society of Japan (in Japanese). 90 (7): 808–12. doi:10.1248/yakushi1947.90.7_808. PMID 5465391.
- ^ Zhao W, Li D, Liu Z, Zheng X, Wang J, Wang E (2013). "Spiclomazine induces apoptosis associated with the suppression of cell viability, migration and invasion in pancreatic carcinoma cells". PLOS ONE. 8 (6): e66362. doi:10.1371/journal.pone.0066362. PMC 3688794. PMID 23840452.
- ^ Michio Nakanishi, Katsuo Arimura, Tatsumi Tsumagari Masa Shiroki, US3574204 (1971 to Yoshitomi Pharmaceutical).
- ^ Katsuo Arimura, Michio Nakanishi, Tadao Okada, US3661902 (1972 to Yoshitomi Pharmaceutical).
- ^ Michio Nakanishi, et al. US3651052 (1972 to Welfide Corp).
