Draft:History of the Cell Cycle
 | Review waiting, please be patient.
This may take 8 weeks or more, since drafts are reviewed in no specific order. There are 1,834 pending submissions waiting for review.
Where to get help
How to improve a draft
You can also browse Wikipedia:Featured articles and Wikipedia:Good articles to find examples of Wikipedia's best writing on topics similar to your proposed article. Improving your odds of a speedy review To improve your odds of a faster review, tag your draft with relevant WikiProject tags using the button below. This will let reviewers know a new draft has been submitted in their area of interest. For instance, if you wrote about a female astronomer, you would want to add the Biography, Astronomy, and Women scientists tags. Editor resources
Reviewer tools
|
The discovery of cells and the cell cycle is critical to our modern understanding of living systems, and the fields of biology, biochemistry, biophysics, and medicine, as well as many others. While Robert Hooke initially discovered cells in 1665, research in the cell cycle has made most of its notable progress in the last two centuries. This includes the first descriptions of mitotic progression, the description of the entire cell cycle including the different parts of interphase, a new molecular understanding of the drivers of the cell cycle, and the discovery of the essential checkpoints.
Discovery of the Cell Cycle and Its Phases
[edit]
Robert Hooke in 1665 coined the term cell to describe the small walled structures he saw within a cork sample[1]. This led to Schleiden and Schwann proposing cell theory that stated all living beings are made of cells and that cells formed de novo by some sort of crystallization, also known as free-cell formation [2]. This was widely accepted for almost three decades until the 1870s where Rudolf Virchow and others showed that cells divide to create new cells[2] [3]. Walther Flemming then began his work which would end up describing in great detail the process of mitosis and mark the beginning of the cell cycle as we know it today[4]. He developed his own methods for staining chromosomes and observed them in cells, documenting their shape and size changes as nuclear division occurred [4] [5] [6]. This led to the two phase cell cycle, mitosis where the nucleus is split, and interphase which encompasses the rest of the time where it appeared to Flemming that the cell was relatively inactive but grew in size.
Research into interphase was not seriously undertaken until the mid 1900s when Alma Howard and Stephen R. Pelc observed the incorporation of of radiolabeled phosphorus into dividing bean cells[7]. They observed that it occurred only in dividing cells and only during interphase. From this, they then observed that there was 3 distinct parts of interphase, G1 (Gap1), S (for DNA synthesis), and G2 (Gap2)[7]. In 1954, Laszlo Lajtha showed bone marrow cells obeyed this 4 stage cycle, and also proposed a G0 (Gap0) phase for cells which exit this cycle and enter a resting phase[8][5]. With this final phase described, the cell cycle as we know today was complete.
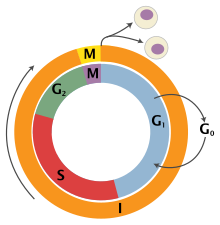
Basic Mechanisms of the Cell Cycle
[edit]In the 1970s, research into how the cells transitioned between these phases began. Many different fields were working on this simultaneously. In cell biology, Rao and Johnson’s fusion experiments gave early evidence for the idea that there was some factor which triggers transitions between phases[9]. They showed that the fusion of a cell in S phase with a cell in G1 will produce an S phase cell, whereas a fusion of an S phase cell and a G2 cell will produce a G2/M phase cell. These imply that there is something which causes a cell to go from G1 to S and another to go from S to G2. They also performed a fusion experiment of an M (mitosis) phase cell and a G1 phase cell which surprisingly led to early progression into mitosis, showing that there must be a third factor that causes cells to enter M phase[9].
In the field of zoology, Yoshio Masui’s lab supported these finding by observing in frog oocytes (Xenopus Laevi) that adding progesterone to prophase arrested oocytes triggered their maturation to metaphase arrest. This, along with their observation that the addition of cytoplasm from a metaphase arrested egg into a prophase arrested oocyte can produce the same maturation in the oocyte, led them to propose the presence of a factor called MPF (Maturation Promoting Factor)[10] in 1971.
At the same time, genetic work in yeast was being done to identify key genes related to the cell cycle. Lee Hartwell took to identifying thermosensitive cell division cycle (cdc) mutants of budding yeast (S. cerevisiae)[11]. These then could be categorized and sequenced to identify the mutated genes. Similarly, Paul Nurse worked with fission yeast (S. pombe) to also identify and sequence cdc mutants[12]. These experiments were able to be performed, as the number of buds or the size of the yeast is directly related to the phase of the cell cycle they were in. From these two different systems, two different cdc mutants were identified to be deficient in cell cycle progression, cdc28 in budding yeast and cdc2 in fission yeast[11][12]. Paul Nurse discovered each of these mutants could be rescued with the other species gene library, leading to the hypothesis that the mechanism to drive the cell cycle is highly conserved and that cdc2/cdc28 could code for that mechanism [12].

After the proposal of MPF, the next challenge was to try and purify this molecule. The successful purification of MPF actually produced two different proteins, one 34kDa and one 45kDa [5]. The former turned out to be the cdc2 equivalent in Xenopus Laevi, however the identity of the other protein remained unknown[5]. While this work was ongoing, Tim Hunt was working on protein synthesis and degradation during the cell cycle, and had identified a cycling protein he named cyclin[13]. Cyclin was synthesized before each cell division and then completely degraded after the cells exited mitosis. Once this was identified, it was quickly found that it associates closely with cdc2, and thus the 45kDa protein was identified[5]. This completed the picture of how the cell cycle is driven, the cdc2-type kinase is present throughout the cell cycle while the cyclin levels fluctuate, this allows the cdc2 kinase to activate and deactivate when needed to transition between phases of the cell cycle. Since then, there have been other cdc kinases and cyclins found in other organisms and some organisms have multiple versions of each. The cdc kinases were then renamed CDKs for cyclin-dependent kinases[5].
Cell Cycle Checkpoints
[edit]Now that the logic of the cell cycle and some of the major factors in driving it had been identified, how these transitions were regulated was the main focus of research. The idea of checkpoints and that each phase is dependent on the previous one being sufficiently completed was proposed by Lee Hartwell in the 1970s[14][15]. This led to the discovery of the 4 main checkpoints in the cell cycle. The G1/S transition was described first by Howard Temin in the early 1971 in chicken embryos and then further elucidated by Arthur Pardee in 1974[5]. It ensures proper that the growth has occurred and that there are sufficient nutrients present. The G2 or DNA damage checkpoint ensures there is no damage to the DNA after replication, and was described by Lee Hartwell in 1989. He identified rad9 as one of the essential genes in the arrest to repair DNA damage[16]. The last two checkpoints both occur in M phase. The first is the mitotic checkpoint, and requires that the spindle is formed properly. This was discovered in the 1970s by Raymond Zrikle[5]. The last checkpoint was discovered in 2006 by Yves Barral and is the cytokinetic checkpoint and determines whether the cell divides[17].
In 2001, the Nobel Prize in Physiology or Medicine was awarded to Lee Hartwell, Paul Nurse, and Tim Hunt for their discoveries and work with checkpoints, CDKs, and cyclins respectively[5].
References
[edit]- ^ Hooke, R. Micrographia, or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses, with Observations and Inquiries Thereupon; Library of Congress: London, UK, 1665.
- ^ a b National Geographic Society. (2019, May 22). "History of the Cell: Discovering the Cell". Retrieved December 12, 2024.
- ^ Wang, Zhixiang. “Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling.” Cells vol. 10,12 3327. 26 Nov. 2021, doi:10.3390/cells10123327
- ^ a b Flemming, W. Zellsubstanz, Kern und Zelltheilung; Von Verlag, F.C.W., Ed.; Vogel: Leipzig, Germany, 1882.
- ^ a b c d e f g h i Uzbekov R, Prigent C. A Journey through Time on the Discovery of Cell Cycle Regulation. Cells. 2022.
- ^ Paweletz, Neidhard. "Walther Flemming: Pioneer of Mitosis Research." Nature Reviews Molecular Cell Biology, vol. 2, no. 1, 2001
- ^ a b HOWARD, A, and S R PELC. “Synthesis of nucleoprotein in bean root cells.” Nature vol. 167,4250 (1951): 599-600. doi:10.1038/167599a0
- ^ Lajtha, L., Oliver, R. & Ellis, F. Incorporation of 32P and Adenine 14C into DNA by Human Bone Marrow Cells In Vitro. Br J Cancer 8, 367–379 (1954). doi.:10.1038/bjc.1954.38
- ^ a b Rao, P N, and R T Johnson. “Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis.” Nature vol. 225,5228 (1970): 159-64. doi:10.1038/225159a0
- ^ Masui, Y. and Markert, C.L., "Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes." J. Exp. Zool., 177 (1971): 129-145.doi:10.1002/jez.1401770202
- ^ a b Hartwell LH. "Macromolecule Synthesis in Temperature-sensitive Mutants of Yeast." J Bacteriol 93 (1967) doi:10.1128/jb.93.5.1662-1670.1967
- ^ a b c Nurse, P., Thuriaux, P. & Nasmyth, K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe . Molec. Gen. Genet. 146, 167–178 (1976). doi:10.1007/BF00268085
- ^ Evans, T et al. “Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division.” Cell vol. 33,2 (1983): 389-96. doi:10.1016/0092-8674(83)90420-8
- ^ Leland H. Hartwell et al., Genetic Control of the Cell Division Cycle in Yeast.Science183,46-51(1974).DOI:10.1126/science.183.4120.46
- ^ Hartwell, L H, and T A Weinert. “Checkpoints: controls that ensure the order of cell cycle events.” Science (New York, N.Y.) vol. 246,4930 (1989): 629-34. doi:10.1126/science.2683079
- ^ Weinert, T, and L Hartwell. “Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae.” Journal of cell science. Supplement vol. 12 (1989): 145-8. doi:10.1242/jcs.1989.supplement_12.12
- ^ Norden, Caren, et al. "The NoCut Pathway Links Completion of Cytokinesis to Spindle Midzone Function to Prevent Chromosome Breakage." Cell, vol. 125, no. 1, 2006, pp. 85-98, doi:10.1016/j.cell.2006.01.045.
