Deruxtecan
Appearance
| |
| Names | |
|---|---|
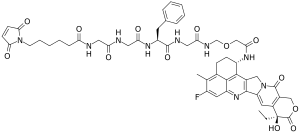
| IUPAC name
6-(2,5-dioxopyrrol-1-yl)-N-[2-[[2-[[(2S)-1-[[2-[[2-[[(10S,23S)-10-ethyl-18-fluoro-10-hydroxy-19-methyl-5,9-dioxo-8-oxa-4,15-diazahexacyclo[14.7.1.02,14.04,13.06,11.020,24]tetracosa-1,6(11),12,14,16,18,20(24)-heptaen-23-yl]amino]-2-oxoethoxy]methylamino]-2-oxoethyl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]amino]-2-oxoethyl]hexanamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C52H56FN9O13 | |
| Molar mass | 1034.068 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Deruxtecan is a chemical compound and a derivative of exatecan that acts as topoisomerase I inhibitor.[1]
It is available linked to specific monoclonal antibody (antibody–drug conjugate), such as:
- Trastuzumab deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma.[2]
- Patritumab deruxtecan, an experimental antibody–drug conjugate to treat non-small-cell lung cancer.[3][4][5]
- Ifinatamab deruxtecan, an experimental anti-cancer treatment.[6][7]
References
[edit]- ^ "A HER2-Targeting Antibody–Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model" (PDF). Archived (PDF) from the original on 24 September 2020. Retrieved 23 December 2019.
- ^ "FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies". U.S.Food and Drug Administration (FDA) (Press release). 20 December 2019. Archived from the original on 20 December 2019. Retrieved 20 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Yu, H.A.; Baik, C.S.; Gold, K.; Hayashi, H.; Johnson, M.; Koczywas, M.; Murakami, H.; Nishio, M.; Steuer, C.; Su, W-C.; Yang, J.; Karam, S.; Qi, Z.; Qiu, Y.; Chen, S.; Yu, C.; Jänne, P.A. (September 2020). "LBA62 Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER3 directed antibody drug conjugate, in patients (pts) with EGFR-mutated (EGFRm) NSCLC". Annals of Oncology. 31: S1189–S1190. doi:10.1016/j.annonc.2020.08.2295. S2CID 225184933.
- ^ Krop, Ian E.; Masuda, Norikazu; Mukohara, Toru; Takahashi, Shunji; Nakayama, Takahiro; Inoue, Kenichi; Iwata, Hiroji; Toyama, Tatsuya; Yamamoto, Yutaka; Hansra, Damien Mikael; Takahashi, Masato; Osaki, Akihiko; Koyama, Kumiko; Inoue, Tatsuya; Yonekura, Takatoshi; Mostillo, Joseph; Ohwada, Shoichi; Tanaka, Yoshimi; Sternberg, David W.; Yonemori, Kan (1 June 2022). "Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC)". Journal of Clinical Oncology. 40 (16_suppl): 1002. doi:10.1200/JCO.2022.40.16_suppl.1002. S2CID 249454563.
- ^ Jänne, Pasi A.; Baik, Christina; Su, Wu-Chou; Johnson, Melissa L.; Hayashi, Hidetoshi; Nishio, Makoto; Kim, Dong-Wan; Koczywas, Marianna; Gold, Kathryn A.; Steuer, Conor E.; Murakami, Haruyasu; Yang, James Chih-Hsin; Kim, Sang-We; Vigliotti, Michele; Shi, Rong; Qi, Zhenhao; Qiu, Yang; Zhao, Lihui; Sternberg, David; Yu, Channing; Yu, Helena A. (1 January 2022). "Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor–Resistant, EGFR -Mutated Non–Small Cell Lung Cancer". Cancer Discovery. 12 (1): 74–89. doi:10.1158/2159-8290.CD-21-0715. PMC 9401524. PMID 34548309.
- ^ Katsumata, Len; Deguchi, Tsuneo; Hasegawa, Jun; Yamato, Michiko; Nishiya, Yumi; Watanabe, Akiko; Honda, Tomoyo; Minami, Megumi; Kasanuki, Naomi; Maejima, Takanori; Muramatsu, Sumie; Herrmann, Monika; Schulte, Nina; Polier, Gernot; Wang, Xuya; Yoshida, Takanori; Izumi, Nanae; Qian, Xiaozhong; Agatsuma, Toshinori; Nakamaru, Kenji (4 April 2023). "Abstract 4891: Ifinatamab deruxtecan (I-DXd), a novel B7-H3-targeting antibody-drug conjugate, demonstrates efficient payload delivery into tumor through target-dependent internalization". Cancer Research. 83 (7_Supplement): 4891. doi:10.1158/1538-7445.AM2023-4891. S2CID 259620618.
- ^ Patel, M.R.; Doi, T.; Koyama, T.; Falchook, G.S.; Friedman, C.F.; Piha-Paul, S.A.; Gutierrez, M.; Awad, M.M.; Mattour, A.H.; Satoh, T.; Okamoto, N.; Singh, J.; Yoshizuka, N.; Qian, M.; Qian, X.; Tran, B.P.; Dosunmu, O.; Lu, P.; Johnson, M.L. (October 2023). "690P Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with advanced solid tumors: Updated clinical and biomarker results from a phase I/II study". Annals of Oncology. 34: S481–S482. doi:10.1016/j.annonc.2023.09.1876. S2CID 264385889.
