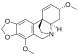
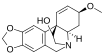
Crinine
Appearance
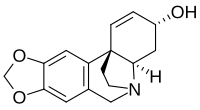
| |
| Names | |
|---|---|
| IUPAC name
(1S,13R,15R)-5,7-dioxa-12-azapentacyclo[10.5.2.01,13.02,10.04,8]nonadeca-2,4(8),9,16-tetraen-15-ol
| |
| Other names
Crinidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H17NO3 | |
| Molar mass | 271.316 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Crinine in an alkaloid found in a variety of plants including many species in the family Amaryllidaceae. It was first isolated and characterized in the 1950s as a constituent of several plants in the genus Crinum, from which it derives its name.[1][2][3]
Several laboratory syntheses of crinine have been reported.[4][5][6][7]
Related compounds
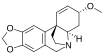
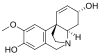
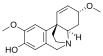
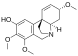
[edit]There are many alkaloids with a chemical structure similar to crinine and they are generally referred to as crinine-type alkaloids. As of 2020, there were at least 85 known crinine-type alkaloids.[8]
Chemical structures of selected crinine-type alkaloids
References
[edit]- ^ Boit, Hans-G. (1954). "Über die Alkaloide von Nerine sarniensis, Crinum Moorei, Hippeastrum vittatum und Clivia miniata (VI. Mitteil. über Amaryllidaceen-Alkaloide)". Chemische Berichte. 87 (11): 1704–1707. doi:10.1002/cber.19540871115.
- ^ Mason, L. H.; Puschett, E. R.; Wildman, W. C. (1955). "Alkaloids of the Amaryllidaceae. IV. Crystalline Alkaloids of Ammocharis coranica (Ker-Gawl.) Herb., Brunsvigia rosea (Lam.) Hannibal and Two Crinum Species". Journal of the American Chemical Society. 77 (5): 1253–1256. Bibcode:1955JAChS..77.1253M. doi:10.1021/ja01610a046.
- ^ Boit, Hans-G.; Ehmke, Horst (1955). "Alkaloide von Sprekelia formosissima, Galanthus Elwesii, Zephyranthes candida und Crinum Powellii (VIII. Mitteil. über Amaryllidaceen-Alkaloide1))". Chemische Berichte. 88 (10): 1590–1594. doi:10.1002/cber.19550881019.
- ^ Muxfeldt, Hans; Schneider, Richard S.; Mooberry, Jared B. (1966). "A Total Synthesis of (±)-Crinine". Journal of the American Chemical Society. 88 (15): 3670–3671. Bibcode:1966JAChS..88.3670M. doi:10.1021/ja00967a052.
- ^ Martin, Stephen F.; Campbell, Carlton L. (1988). "Total syntheses of (.+-.)-crinine and (.+-.)-buphanisine". The Journal of Organic Chemistry. 53 (14): 3184–3190. doi:10.1021/jo00249a011.
- ^ Whitlock, Howard W.; Smith, Gary Lee. (1967). "Total synthesis of DL-crinine". Journal of the American Chemical Society. 89 (14): 3600–3606. Bibcode:1967JAChS..89.3600W. doi:10.1021/ja00990a045.
- ^ Bru, Claire; Guillou, Catherine (2006). "Total syntheses of crinine and related alkaloids". Tetrahedron. 62 (38): 9043–9048. doi:10.1016/j.tet.2006.07.005.
- ^ Strahil Berkov, Jaume Bastida (2020). "Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids". The Alkaloids: Chemistry and Biology.