Bis(2,4-di-tert-butylphenyl) pentaerythritol diphosphite
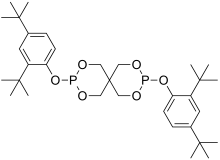
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,9-bis(2,4-di-tert-butylphenoxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane | |
| Other names
Trade names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.043.578 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C33H50O6P2 | |
| Molar mass | 604.705 g·mol−1 |
| Appearance | White solid |
| Density | 1.166 at 20°C |
| Melting point | 172–179 °C (342–354 °F; 445–452 K) |
| Boiling point | 311 °C (592 °F; 584 K) |
| 93.0 µg/L at 25°C | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H410 | |
| P261, P264, P264+P265, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bis(2,4-di-tert-butylphenyl) pentaerythritol diphosphite is an organophosphite used as a polymer stabilizer in plastics. Like other phosphite antioxidants it primarily acts to remove hydroperoxides and is typically used in combination with hindered phenolic antioxidants such as pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate).
Synthesis
[edit]It is formed by a reaction between phosphorous trichloride, pentaerythritol and 2,4-di-tert-butylphenol. The poor solubility of pentererythritol can be an issue and non-nuncleophilic amines are often used to promote the reaction.[1]
Properties and applications
[edit]Compared to tris(2,4-di-tert-butylphenyl)phosphite (a common phosphite antioxidant) it has higher activity but lower stability against hydrolysis. Trace levels of amine bases are often added to commercial material to slow hydrolysis and increase storage life.[2] It's crystal structure has been determined.[3] It is comparable with a wide range of plastics including polyolefins, engineering plastics and polyurethane fibers. It is an approved food contact material in the US.[4]
References
[edit]- ^ Zhu, Yuliang; Liu, Xinyue; Tang, Ying; Xu, Kexin; Tang, Xin; Zhu, Longzhi; Xiong, Biquan (13 November 2024). "Recent Advances in the Synthesis of Commercially Available Phosphite Antioxidants". ChemistryOpen: e202400135. doi:10.1002/open.202400135. PMID 39538976.
- ^ Papanastasiou, M.; McMahon, A.W.; Allen, N.S.; Doyle, A.M.; Johnson, B.J.; Keck-Antoine, K. (November 2006). "The hydrolysis mechanism of bis(2,4-di-tert-butyl)pentaerythritol diphosphite (Alkanox P24): An atmospheric pressure photoionisation mass spectrometric study". Polymer Degradation and Stability. 91 (11): 2675–2682. doi:10.1016/j.polymdegradstab.2006.04.023.
- ^ Barren, J. P.; Bryant, G. L.; Garbauskas, M. F.; Mahood, J. A. (15 August 1995). "Bis(2,4-di-tert-butylphenyl) Pentaerythritol Diphosphite". Acta Crystallographica Section C Crystal Structure Communications. 51 (8): 1636–1639. Bibcode:1995AcCrC..51.1636B. doi:10.1107/S0108270195000965.
- ^ "FDA broadens food contact approval for SI Group's Ultranox 626 antioxidant". Additives for Polymers. 2019 (9): 4. September 2019. doi:10.1016/S0306-3747(19)30174-5.
