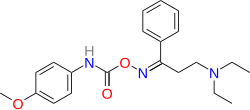
Anidoxime
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | Bamoxine, BRL 11870, E 142, USV-E 142. |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H27N3O3 |
| Molar mass | 369.465 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Anidoxime was an experimental drug that had been evaluated for use as an oral analgesic.[1][2]
The name is an eponym of an anisdine group and an oxime. The analgesic properties are reportedly equipotent to or greater than morphine but the physical dependence liability may be less.[3]
In rats, the major metabolic pathway is hydrolysis of the carbamoyl group followed by decarboxylation.[4]
References
[edit]- ^ Grainger DJ, Gawley TH, Dundee JW (March 1977). "Anidoxime: a clinical trial of an oral analgesic agent". British Journal of Anaesthesia. 49 (3): 257–8. doi:10.1093/bja/49.3.257. PMID 334207.
- ^ Serradell MN, Blancafort P (1978). "Anidoxime hydrochloride". Drugs of the Future. 3 (3): 173. doi:10.1358/dof.1978.003.03.1000263.
- ^ Watzman N, Buckley JP (February 1980). "Comparative analgesic, behavioral, and dependence properties of morphine and O-(4-methoxylphenylcarbamoyl)-3-diethylaminopropiophenone oxime hydrochloride". Journal of Pharmaceutical Sciences. 69 (2): 225–6. doi:10.1002/jps.2600690234. PMID 7188962.
- ^ Gollamudi R, Glassman JM (February 1982). "Tissue distribution and metabolism of O-(4-methoxyphenylcarbamoyl)-3-diethylaminopropiophenone oxime hydrochloride (USVP-E142) in the rat". Journal of Applied Toxicology. 2 (1): 21–26. doi:10.1002/jat.2550020106.
