Ammonium hexafluorotitanate
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.291 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F6H8N2Ti | |
| Molar mass | 197.935 g·mol−1 |
| Appearance | white solid |
| Density | 1.675g/cm3 |
| Related compounds | |
Other anions
|
Hexafluorosilicate Hexafluorotitanic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
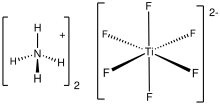
Ammonium hexafluorotitanate is the inorganic compound with the chemical formula (NH4)2[TiF6]. A colorless salt, the compound consists of ammonium ions and the hexafluorotitanate dianion.
Synthesis
[edit]The compound is encountered in the extraction of titanium from its principal ore ilmenite: the ore is treated with excess ammonium fluoride:[1]
- FeTiO3 + 10 NH4F → (NH4)2FeF4 + (NH4)2TiF6 + 6 H2O
After removal of iron impurities, the titanium is recovered as a hydrated titanium dioxide by treatment of the aqueous extract of the hexafluoride with ammonia:
- (NH4)2TiF6 + 4 NH3 + 2 H2O → TiO2 + 6 NH4F
Structure
[edit]Many salts of hexafluorotitanate have been characterized by X-ray crystallography. In the lattice [TiF6]2- octahedra interact with the ammonium cations by hydrogen bonds.[2]
References
[edit]- ^ Bichowsky, Foord Von (1957). "Extraction of Titanium(IV) Oxide from Ilmenite". Inorganic Syntheses. Vol. V. pp. 79–82. doi:10.1002/9780470132364.ch22. ISBN 9780470132364.
- ^ Tun, Z.; Brown, I. D. (1982). "Hydrogen bonding in Diammonium Hexafluorotitanate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 38 (6): 1792–1794. Bibcode:1982AcCrB..38.1792T. doi:10.1107/S0567740882007195.
