Aluminium chloride
 Aluminium trichloride hexahydrate, pure (top), and contaminated with iron(III) chloride (bottom)
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Aluminium chloride
| |||
| Other names
Aluminium(III) chloride
Aluminium trichloride Trichloroaluminum | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.371 | ||
| EC Number |
| ||
| 1876 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| AlCl3 | |||
| Molar mass |
| ||
| Appearance | Colourless crystals, hygroscopic | ||
| Density |
| ||
| Melting point | |||
| |||
| Solubility |
| ||
| Vapor pressure |
| ||
| Viscosity |
| ||
| Structure | |||
| Monoclinic, mS16 | |||
| C12/m1, No. 12[3] | |||
a = 0.591 nm, b = 0.591 nm, c = 1.752 nm[3]
| |||
Lattice volume (V)
|
0.52996 nm3 | ||
Formula units (Z)
|
6 | ||
| Octahedral (solid) Tetrahedral (liquid) | |||
| Trigonal planar (monomeric vapour) | |||
| Thermochemistry | |||
Heat capacity (C)
|
91.1 J/(mol·K)[4] | ||
Std molar
entropy (S⦵298) |
109.3 J/(mol·K)[4] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−704.2 kJ/mol[4] | ||
Gibbs free energy (ΔfG⦵)
|
−628.8 kJ/mol[4] | ||
| Pharmacology | |||
| D10AX01 (WHO) | |||
| Hazards | |||
| GHS labelling:[6] | |||

| |||
| Danger | |||
| H314 | |||
| P260, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338+P310, P310 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
380 mg/kg, rat (oral, anhydrous) 3311 mg/kg, rat (oral, hexahydrate) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
None[5] | ||
REL (Recommended)
|
2 mg/m3[5] | ||
IDLH (Immediate danger)
|
N.D.[5] | ||
| Related compounds | |||
Other anions
|
|||
Other cations
|
|||
Related Lewis acids
|
|||
| Supplementary data page | |||
| Aluminium chloride (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Topical |
| ATC code |
|
| Identifiers | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.371 |
| Data page | |
| Aluminium chloride (data page) | |
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.
The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry.[7] The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
History
[edit]The salt was known in the 18th century as muriate of alumina, marine alum, argillaceous marine salt,[8] muriated clay.[9] It was first chemically studied in the 1830s.[10]
This section needs expansion. You can help by adding to it. (November 2024) |
Structure
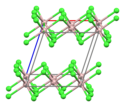
[edit]
Anhydrous
[edit]AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry.[11] Yttrium(III) chloride adopts the same structure, as do a range of other compounds. When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) versus solid aluminium trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapour phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3 monomer, which is structurally analogous to BF3. The melt conducts electricity poorly,[12] unlike more ionic halides such as sodium chloride.
Aluminium chloride monomer belongs to the point group D3h in its monomeric form and D2h in its dimeric form.
Hexahydrate
[edit]The hexahydrate consists of octahedral [Al(H2O)6]3+ cation centers and chloride anions (Cl−) as counterions. Hydrogen bonds link the cation and anions.[13] The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminium ion surrounded by six water ligand molecules. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions.
Uses
[edit]Alkylation and acylation of arenes
[edit]AlCl3 is a common Lewis-acid catalyst for Friedel-Crafts reactions, both acylations and alkylations.[14] Important products are detergents and ethylbenzene. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene.[12] In the general Friedel-Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[14]
The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding. For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed.[15] Detailed procedures are available for alkylation[16] and acylation[17][18] of arenes.
A general problem with the Friedel-Crafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites.[7]
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst.[19]
Other applications in organic and organometallic synthesis
[edit]Aluminium chloride finds a wide variety of other applications in organic chemistry.[20] For example, it can catalyse the ene reaction, such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[21]
It is used to induce a variety of hydrocarbon couplings and rearrangements.[22][23]
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the Fischer–Hafner synthesis. Dichlorophenylphosphine is prepared by reaction of benzene and phosphorus trichloride catalyzed by aluminium chloride.[24]
Medical
[edit]Topical aluminum chloride hexahydrate is used for the treatment of hyperhidrosis (excessive sweating).[25][26][27]
Reactions
[edit]Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene.[14] It forms tetrachloroaluminate ([AlCl4]−) in the presence of chloride ions.
Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydroaluminates.[citation needed]
Reactions with water
[edit]Anhydrous aluminium chloride is hygroscopic, having a very pronounced affinity for water. It fumes in moist air and hisses when mixed with liquid water as the Cl− ligands are displaced with H2O molecules to form the hexahydrate [Al(H2O)6]Cl3. The anhydrous phase cannot be regained on heating the hexahydrate. Instead HCl is lost leaving aluminium hydroxide or alumina (aluminium oxide):
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands:
- [Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+
Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with dilute sodium hydroxide:
- AlCl3 + 3 NaOH → Al(OH)3 + 3 NaCl
Synthesis
[edit]Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 and 750 °C (1,202 and 1,382 °F).[12]
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminium chloride may be formed via a single displacement reaction between copper(II) chloride and aluminium.
- 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.[7]
Hydrated aluminium trichloride is prepared by dissolving aluminium oxides in hydrochloric acid. Metallic aluminium also readily dissolves in hydrochloric acid ─ releasing hydrogen gas and generating considerable heat. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated:
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.[12]
Natural occurrence
[edit]Anhydrous aluminium chloride is not found as a mineral. The hexahydrate, however, is known as the rare mineral chloraluminite.[28] A more complex, basic and hydrated aluminium chloride mineral is cadwaladerite.[29][28]
Safety
[edit]Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required. It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact.[30]
See also
[edit]References
[edit]- ^ a b c d Haynes WM, ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.45. ISBN 1-4398-5511-0.
- ^ a b "Properties of substance: Aluminium chloride". Chemister.ru. 2007-03-19. Archived from the original on 2014-05-05. Retrieved 2017-03-17.
- ^ a b Ketelaar JA (1935). "Die Kristallstruktur der Aluminiumhalogenide II". Zeitschrift für Kristallographie – Crystalline Materials. 90 (1–6): 237–255. doi:10.1524/zkri.1935.90.1.237. S2CID 100796636.
- ^ a b c d Haynes WM, ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 5.5. ISBN 1-4398-5511-0.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0024". National Institute for Occupational Safety and Health (NIOSH).
- ^ Sigma-Aldrich Co., Aluminium chloride.
- ^ a b c Helmboldt O, Keith Hudson L, Misra C, Wefers K, Heck W, Stark H, et al. (2007). "Aluminum Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_527.pub2. ISBN 978-3527306732.
- ^ FOURCROY AF (1790). Elements of natural history, and of chemistry: being the second edition of the elementary lectures on those sciences ... enlarged and improved by the author ... Translated into English, with ... notes; and an historical preface by the translator W. Nicholson.
- ^ Berthollet CL (1791). Elements of the Art of Dyeing ... Translated ... by William Hamilton. Stephen Couchman; sold by J. Johnson.
- ^ Gambold M (1835). The American Journal of Science. Kline Geology Laboratory, Yale University.
- ^ Wells AF (1984). Structural Inorganic Chemistry. Oxford, United Kingdom.: Oxford Press. ISBN 0198553706.
In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions.
- ^ a b c d Greenwood NN, Earnshaw A (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- ^ Andress KR, Carpenter C (1934). "Kristallhydrate II. Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat". Zeitschrift für Kristallographie – Crystalline Materials. 87. doi:10.1524/zkri.1934.87.1.446. S2CID 263857074.
- ^ a b c Olah GA, ed. (1963). Friedel-Crafts and Related Reactions. Vol. 1. New York City: Interscience.
- ^ Nenitzescu CD, Cantuniari IP (1933). "Durch Aluminiumchlorid Katalysierte Reaktion, VI. Mitteil.: Die Umlagerung des Cyclohexans in Metyl-cyclopentan". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 66 (8): 1097–1100. doi:10.1002/cber.19330660817. ISSN 1099-0682.
- ^ Reeves JT, Tan Z, Fandrick DR, Song JJ, Yee NK, Senanayake CH (2012). "Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one". Organic Syntheses. 89: 210. doi:10.15227/orgsyn.089.0210.
- ^ Paruch K, Vyklicky L, Katz TJ (2003). "Preparation of 9,10-Dimethoxyphenanthrene and 3,6-Diacetyl-9,10-Dimethoxyphenanthrene". Organic Syntheses. 80: 227. doi:10.15227/orgsyn.080.0227.
- ^ Seed AJ, Sonpatki V, Herbert MR (2002). "3-(4-Bromobenzoyl)propanoic Acid". Organic Syntheses. 79: 204. doi:10.15227/orgsyn.079.0204.
- ^ Wade LG (2003). Organic Chemistry (5th ed.). Upper Saddle River, New Jersey: Prentice Hall. ISBN 013033832X.
- ^ Galatsis P (1999). "Aluminum Chloride". In Reich HJ, Rigby JH (eds.). Acidic and Basic Reagents. Handbook of Reagents for Organic Synthesis. New York City: Wiley. pp. 12–15. ISBN 978-0-471-97925-8.
- ^ Snider BB (1980). "Lewis-acid catalyzed ene reactions". Acc. Chem. Res. 13 (11): 426. doi:10.1021/ar50155a007.
- ^ Rieke RD, Bales SE, Hudnall PM, Burns TP, Poindexter GS (1979). "Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornanecarboxylic Acid". Organic Syntheses. 59: 85. doi:10.15227/orgsyn.059.0085.
- ^ Shama SA, Wamser CC (1983). "Hexamethyl Dewar Benzene". Organic Syntheses. 61: 62. doi:10.15227/orgsyn.061.0062.
- ^ Buchner B, Lockhart Jr LB (1951). "Phenyldichlorophosphine". Organic Syntheses. 31: 88. doi:10.15227/orgsyn.031.0088.
- ^ McConaghy JR, Fosselman D (June 2018). "Hyperhidrosis: Management Options". American Family Physician. 97 (11): 729–734. PMID 30215934.
- ^ Nawrocki S, Cha J (September 2019). "The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options". Journal of the American Academy of Dermatology. 81 (3): 669–680. doi:10.1016/j.jaad.2018.11.066. PMID 30710603.
- ^ "Aluminum Chloride (Topical) (Monograph)". American Society of Health System Pharmacists (ASHP). drugs.com.
- ^ a b "List of Minerals". www.ima-mineralogy.org. International Mineralogical Association. March 21, 2011.
- ^ "Cadwaladerite". www.mindat.org.
- ^ Aluminium Chloride. solvaychemicals.us