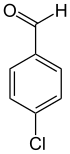
4-Chlorobenzaldehyde
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| 385858 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.953 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H5ClO | |
| Molar mass | 140.57 g·mol−1 |
| Melting point | 47.5 °C (117.5 °F; 320.6 K) |
| Boiling point | 213.5 °C (416.3 °F; 486.6 K) |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Warning | |
| H302, H315, H317, H319, H411 | |
| P261, P264, P264+P265, P270, P271, P272, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P333+P313, P337+P317, P362+P364, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Chlorobenzaldehyde (o-Chlorobenzaldehyde) is an organic compound with the formula ClC5H4CHO. It is one of three isomeric monochlorinated benzaldehydes.
Preparation
[edit]It can be produced by hydrolysis of 4-chlorobenzal chloride:[2]
- ClC5H4CHCl2 + H2O → ClC5H4CHO + 2 HCl
It can also be produced by the oxidation of 4-chlorobenzyl alcohol.[3][4] It can be further oxidized to 4-chlorobenzoic acid.[5] It will react with malononitrile to form 4-chlorobenzylidenylmalononitrile. [6]4-Chlorobenzaldehyde reacts with benzylamine to produce N-(4-chlorobenzylidenyl)benzylamine。[7]
References
[edit]- ^ "4-Chlorobenzaldehyde". pubchem.ncbi.nlm.nih.gov. Retrieved 3 April 2022.
- ^ Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3-527-30385-4.
- ^ Yang, Y. X.; An, X. Q.; Kang, M.; Zeng, W.; Yang, Z. W.; Ma, H. C. (2020). "A Simple and Effective Method for Catalytic Oxidation of Alcohols Using the Oxone/Bu4NHSO4 Oxidation System". Russian Journal of Organic Chemistry. 56 (3): 521–523. doi:10.1134/S1070428020030240. ISSN 1070-4280. S2CID 216049138. Retrieved 2021-05-10.
- ^ Alegre-Requena, Juan V.; Marqués-López, Eugenia; Herrera, Raquel P. (2018-01-04). "Organocatalyzed Enantioselective Aldol and Henry Reactions Starting from Benzylic Alcohols". Advanced Synthesis & Catalysis. 360 (1): 124–129. doi:10.1002/adsc.201701351. hdl:10261/186549. ISSN 1615-4150. S2CID 102499775. Retrieved 2021-05-10.
- ^ Hajimohammadi, Mahdi; Azizi, Naeleh; Tollabimazraeno, Sajjad; Tuna, Ali; Duchoslav, Jiri; Knör, Günther (2021). "Cobalt (II) Phthalocyanine Sulfonate Supported on Reduced Graphene Oxide (RGO) as a Recyclable Photocatalyst for the Oxidation of Aldehydes to Carboxylic Acids". Catalysis Letters. 151 (1): 36–44. doi:10.1007/s10562-020-03287-9. ISSN 1011-372X. S2CID 220312197. Retrieved 2021-05-10.
- ^ Gao, Ziyang; Yang, Hongyuan; Liu, Qing (2019). "Natural Seashell Waste as an Efficient and Low-Cost Catalyst for the Synthesis of Arylmethylenemalonitriles". CLEAN – Soil, Air, Water. 47 (10): 1900129. Bibcode:2019CSAW...4700129G. doi:10.1002/clen.201900129. ISSN 1863-0650. S2CID 203946880. Retrieved 2021-05-10.
- ^ He, Meixia; Lehn, Jean-Marie (2019-11-20). "Time-Dependent Switching of Constitutional Dynamic Libraries and Networks from Kinetic to Thermodynamic Distributions". Journal of the American Chemical Society. 141 (46): 18560–18569. Bibcode:2019JAChS.14118560H. doi:10.1021/jacs.9b09395. ISSN 0002-7863. PMID 31714075. S2CID 207938797. Retrieved 2021-05-10.
