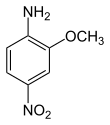
2-Methoxy-4-nitroaniline
Appearance
| |
| Names | |
|---|---|
| Other names
4-Nitro-o-anisidine, 4-Nitroanisidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.354 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H8N2O3 | |
| Molar mass | 168.152 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.42 g/cm3[1] |
| Melting point | 140–142 °C (284–288 °F; 413–415 K) |
| Hazards | |
| GHS labelling:[1] | |
  
| |
| Warning | |
| H302, H351, H411 | |
| P203, P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P318, P319, P321, P330, P332+P317, P337+P317, P362+P364, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methoxy-4-nitroaniline is an organic compound with the formula O2NC6H3(OCH3)NH2.[2] It is one of four isomers of methoxynitroaniline. The compound is a precursor to the commercial Pigment Yellow 74.
Safety
[edit]It induces expression of some versions of cytochrome P450.[3]
References
[edit]- ^ Mehdi, S. (1992). "Structure of 2-methoxy-4-nitroaniline". Acta Crystallographica Section C Crystal Structure Communications. 48 (4): 749–751. Bibcode:1992AcCrC..48..749M. doi:10.1107/S0108270191010739.
- ^ Bao, Yuxin; Wu, Jiaxin; Zhao, Xi; Zhao, Hongkun (2020). "2-Methoxy-4-nitroaniline Solubility in Several Monosolvents: Measurement, Correlation, and Solvent Effect Analysis". Journal of Chemical & Engineering Data. 65 (2): 757–765. doi:10.1021/acs.jced.9b00991.
- ^ Degawa, Masakuni; Nakayama, Masafumi; Konno, Yoshihiro; Masubuchi, Kazuhiro; Yamazoe, Yasushi (1998). "2-Methoxy-4-nitroaniline and its isomers induce cytochrome P4501A (CYP1A) enzymes with different selectivities in the rat liver". Biochimica et Biophysica Acta (BBA) - General Subjects. 1379 (3): 391–398. doi:10.1016/S0304-4165(97)00118-9. PMID 9545601.
