2,5-Undecanedione
 Structural formula of 2,5-Undecanedione
| |
| Names | |
|---|---|
| Other names
Undecan-2,5-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.027.544 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H20O2 | |
| Molar mass | 184.279 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,5-Undecanedione is a γ-diketone in which the two carbonyl groups are separated by two methylene groups.
Synthesis
[edit]Heinz Hunsdiecker described the synthesis of 2,5-undecanedione from 5-methylfurfural in 1942. 5-Methylfurfural 1 is first reacted with methyl propyl ketone 2 in an aldol condensation reaction to form an α,β-unsaturated carbonyl compound 3. The double bond of the side chain condensation product is reduced with sodium amalgam. The intermediate 4 is then converted to 2-methyl-5-hexylfuran 5 in a Wolff-Kishner reduction using hydrazine. An acid-catalyzed reaction at 120 °C (248 °F) cleaves the furan ring, producing 2,5-undecanedione 6.[1]
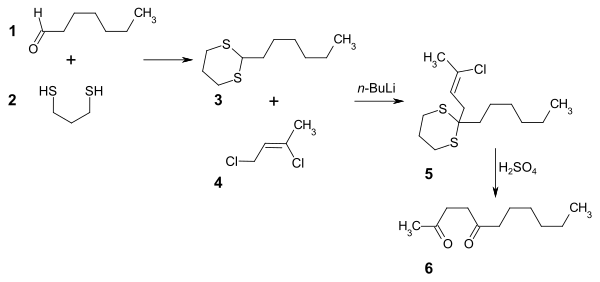
An alternative route of synthesis starts with heptanal 1. First, the carbonyl group is protected with 1,3-propanedithiol 2, producing a dithiane 3. The dithiane is deprotonated using n-Butyllithium in tetrahydrofuran, and alkylated using 1,3-dichloro-2-butene 4 to give the intermediate 5. Hydrolysis of the dithioacetal protecting group with concentrated sulfuric acid yields 2,5-undecanedione 6, which can be worked up and purified using Girard's reagent T[2] in methanol.[3]
Uses
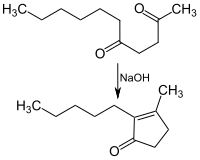
[edit]2,5-Undecanedione is used in the Hunsdiecker condensation, a reaction developed by Heinz Hunsdiecker in 1942 to produce cyclopentenones from γ-ketones, in sodium hydroxide solution to produce the fragrance dihydrojasmone:[1]
References
[edit]- ^ a b Heinz Hunsdiecker (1942-05-06), "Über das Verhalten der γ-Diketone, I. Mitteilung", Berichte der Deutschen Chemischen Gesellschaft, vol. 75, no. 5, pp. 447–454, doi:10.1002/cber.19420750502
- ^ PubChem. "Girard's reagent T". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-11-05.
- ^ Ho, Tse-Lok; Ho, Honor C.; Wong, C. M. (1973-01-15). "A Synthetic Route to Dihydrojasmone; Sulfuric Acid as Dethioacetalization Agent". Canadian Journal of Chemistry. 51 (2): 153–155. doi:10.1139/v73-023. ISSN 0008-4042.