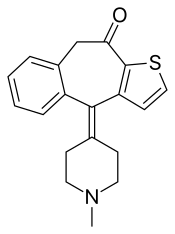
Ketotifen
 | |
| Clinical data | |
|---|---|
| Trade names | Zaditor,[1] Alaway, others |
| Other names | ketotifen fumarate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604033 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, eye drops, drug-eluting contact lenses |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 75% |
| Metabolism | Liver |
| Elimination half-life | 12 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.348 |
| Chemical and physical data | |
| Formula | C19H19NOS |
| Molar mass | 309.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ketotifen is an antihistamine medication and a mast cell stabilizer used to treat allergic conditions such as conjunctivitis, asthma, and urticaria (hives). Ketotifen is available in ophthalmic (eye drops or drug-eluting contact lenses) and oral (tablets or syrup) forms: the ophthalmic form relieves eye itchiness and irritation associated with seasonal allergies, while the oral form helps prevent systemic conditions such as asthma attacks and allergic reactions. In addition to treating allergies, ketotifen has shown efficacy in managing systemic mast cell diseases such as mastocytosis and mast cell activation syndrome (MCAS), which involve abnormal accumulation or activation of mast cells throughout the body. Ketotifen is also used for other allergic-type conditions like atopic dermatitis (eczema) and food allergies.
Ketotifen acts by blocking the H1 histamine receptors, which are found on various cells in the body, such as smooth muscle, endothelium, and nerve cells. This blocking prevents the binding of histamine to these receptors and thus reduces the symptoms of histamine-mediated reactions, such as itching, sneezing, wheezing, and swelling. Ketotifen also prevents the release of histamine and other inflammatory substances from immune cells (mast cells); this action helps reduce symptoms of conditions (including allergic conditions) by blocking the activation of these cells. In addition to its antihistaminic activity, ketotifen also functions as a leukotriene antagonist, which blocks inflammation-causing chemicals known as leukotrienes; it also acts as a phosphodiesterase inhibitor that regulates blood vessel dilation.
Ketotifen can have side effects, including drowsiness, weight gain, dry mouth, irritability, increased nosebleeds when taken orally, and temporary burning or stinging sensations in the eyes when used in the ophthalmic form. Ketotifen has contraindications for individuals with certain medical conditions, such as acute porphyrias or epilepsy. Controversies surrounding ketotifen include its classification as a first-generation or second-generation antihistamine due to varying criteria of classification.
In 2022, it was the 243rd most commonly prescribed medication in the United States, with more than 1 million prescriptions.[6][7]
Medical uses
[edit]Ketotifen, an antihistamine medication and a mast cell stabilizer, is most commonly sold as a salt with fumaric acid, ketotifen fumarate, and is available in two forms:[8]
- in its ophthalmic form (eye drops or drug-eluting contact lenses),[9][5][10] it is used to treat allergic conjunctivitis;[11][1]
- in its oral form (tablets or syrup),[8] it is used to prevent asthma attacks or anaphylaxis,[12][13] as well as various mast cell, allergic-type disorders.[14][15][16]
Ketotifen ophthalmic solution (eye drops) relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. Ketotifen in the form of eye drops has not been studied in children under three years old,[1] whereas drug-eluting contact lenses have not been studied in children under eleven years old.[5]
Drug-eluting contact lenses, which release ketotifen medication, are used to help prevent itchy eyes caused by allergies. The lenses can also correct vision problems like nearsightedness and farsightedness. These lenses are meant for people who don't have red eyes, can comfortably wear contact lenses, and have less than 1 degree of astigmatism.[5]
Oral ketotifen is used to treat asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, chronic urticaria (hives), cold-induced urticaria, cholinergic urticaria, exercise-induced urticaria, systemic mast cell diseases such as mastocytosis and mast cell activation syndrome (MCAS), as well as allergic and nonallergic anaphylaxis. Ketotifen has also shown efficacy in managing angioedema and food allergies.[17]
As an antihistamine medication, ketotifen acts by blocking the H1 histamine receptors,[11] which are found on various cells in the body, such as smooth muscle, endothelium, and nerve cells.[18] This blocking prevents the binding of histamine to these receptors and thus reduces the symptoms of histamine-mediated reactions, such as itching, sneezing, wheezing,[19][20] and swelling.[21]
As a mast cell stabilizer to treat MCAS, oral ketotifen prevents the release of histamine and other inflammatory substances from mast cells, which are immune cells that react to allergens.[17] Therefore, ketotifen, by blocking a calcium channel essential for mast cell activation,[22] helps reduce symptoms of allergic conditions.[17] These allergic conditions include asthma, hay fever, and conjunctivitis caused by mast cell activation.[17] Calcium channels are proteins in mast cell membranes that allow calcium ions to enter the cell, triggering the release of histamine and other inflammatory substances. When these channels open, calcium floods into the cells, causing them to degranulate.[23][24] By blocking these channels, ketotifen prevents this process, reducing allergic reactions.[22] In Canada, Europe, and Mexico, oral ketotifen is commonly prescribed for these indications (asthma, hay fever, and conjunctivitis caused by mast cell activation).[25][16][12] In patients with MCAS, ketotifen reduces episodes of flushing, gastrointestinal symptoms (such as abdominal pain, diarrhea), respiratory symptoms (such as wheezing), and other systemic manifestations. Still, treatment plans for MCAS typically involve a combination of medications targeting different aspects of mast cell activation along with lifestyle modifications to minimize triggers.[17]
The maximum antihistamine and mast cell stabilizing effect of oral ketotifen is achieved on long-term administration, and a period of at least 6-12 weeks is necessary for a maximum therapeutic effect to start.[26] The sedation side effect decreases over time during such long-term administration, but the antihistamine and mast cell stabilizing properties persist even if administered for 12 months or longer.[27]
Oral ketotifen is available at compounding pharmacies in the United States with a prescription requirement, still, the use of oral ketotifen is only approved by the Food and Drug Administration (FDA) for adults and older children with asthma or allergic conditions.[16][12] However, ketotifen eye drops are approved in the US for people who are at least three years of age.[28][29] In the EU, ketotifen oral formulatios (syrup, tables and capsules) are approved by the European Medicines Agency for adult use.[30] In the UK, ketotifen is available as tables and elixir (liquid).[31]
Oral ketotifen can be used as a long-term control medication for asthma and wheeze in children, and it has been shown to improve the control of asthma by reducing the need for bronchodilators, decreasing symptoms, preventing exacerbations, and reducing the use of rescue oral steroids, ketotifen has also been found to be effective when used alone or in combination with other medications. Oral ketotifen is an alternative to inhaled therapy for asthma in children, especially for younger children who may have difficulty using inhalers.[32]
The mean elimination half-life of oral ketotifen is 12 hours.[33] Besides its anti-histaminic activity, it is also a functional leukotriene antagonist[34] (a medication that blocks the action of leukotrienes, which are chemicals that cause inflammation and narrowing of the airways in some allergic and respiratory conditions)[35][36] and a phosphodiesterase inhibitor[37][38] (a medication that blocks the enzymes that regulate the levels of cAMP and cGMP, which are molecules that control blood vessel dilation and smooth muscle relaxation in the body).[39][40]
Contraindications
[edit]The eye drops are contraindicated for individuals who have a known hypersensitivity to ketotifen or any other ingredient in the formulation, whereas drug-eluting contact lenses are contraindicated for those who experience irritation from wearing contact lenses. Eye drops are not recommended for use in children under three years of age,[41][29][42] whereas drug-eluting contact lenses are not recommended for children under eleven years of age.[5]
For oral ketotifen, the contraindication is for known hypersensitivity to any component of the product. Caution should be taken on the following conditions:[11]
- acute porphyrias[43] (a group of rare disorders that occur when the body cannot make enough of a substance called heme, which is needed for red blood cells to carry oxygen, this causes a build-up of chemicals called porphyrins, which can damage the nerves and the skin) - unlike other histamines, ketotifen appears to be relatively safe in acute porphyria, still, caution should be taken[44]
- epilepsy (a disorder causing recurrent seizures) - [45] ketotifen may increase the risk of seizures,[46]
- pyloroduodenal obstruction[47][48][41] (a condition where the passage of food from the stomach to the small intestine is blocked by something, such as a muscle, an ulcer, a tumor, or a gallstone),[49][50]
- susceptibility to angle-closure glaucoma[51] (a condition where the iris, the colored part of the eye, bulges and blocks the drainage of fluid from the eye, causing high pressure and damage to the optic nerve, which connects the eye to the brain),[52] and
- urinary retention (inability to urinate).[41]
The use of ketotifen eye drops during pregnancy and lactation is considered safe, as absorption through the eye is limited. It is unlikely to cause any adverse effects in breastfeeding infants after maternal use. To minimize the amount of medication transferred to breast milk when using eye drops, the National Institute of Child Health and Human Development advises to apply pressure on the tear duct near the corner of the eye for at least one minute and remove any excess solution with a tissue.[53] Ketotifen safety when taken via the oral route (tablets or syrup) during pregnancy and lactation remains unknown; therefore, it is not recommended to use ketotifen orally during these periods until sufficient safety data becomes available.[53]
Side effects
[edit]Common side effects of ophthalmic use are eye redness and swelling. Less common are eye discharge, eye discomfort, eye pain, hives, increased itching of eyes, and rash. Ophthalmic use of ketotifen may also cause burning, stinging, or itching of the eyes, blurred vision, or increased sensitivity to light.[29]
Side effects of systemic (oral) use include drowsiness, weight gain (5.0–5.4 kilograms (11.0–11.9 lb)), dry mouth, irritability, and increased nosebleeds.[54] Systemic use of ketotifen may also cause abdominal pain, nausea, vomiting, constipation, diarrhea, headache, dizziness, or fatigue. In rare cases, systemic use of ketotifen may cause serious side effects such as anaphylaxis, liver dysfunction, blood disorders, or seizures. Systemic use of ketotifen may interact with other drugs that cause sedation, such as alcohol, antihistamines, opioids, benzodiazepines, or antidepressants. Systemic use of ketotifen may affect the results of some laboratory tests, such as skin tests for allergies or blood glucose levels.[8]
Overdose
[edit]The symptoms of ketotifen overdose are dose-dependent and may vary from mild to severe. The onset of symptoms may be delayed for several hours after ingestion, and the duration of symptoms may last for more than 24 hours.[55][56][57]
The most common symptom of ketotifen overdose is significant sedation. Other symptoms may include confusion, disorientation, agitation, hallucinations, ataxia (impairment of voluntary muscle movement), tremor (involuntary regular muscle contraction), myoclonus (involuntary, irregular muscle twitch), nystagmus (dysfunction of eye movement), dysarthria (poor speech), and slurred speech.[55][56][57]
Other symptoms of ketotifen overdose may include tachycardia (fast, pounding, or irregular heartbeat or pulse), hypotension (low blood pressure), convulsions, hyperexcitability (particularly in children), reversible coma, unusual tiredness or weakness, blurred vision, dizziness or fainting, loss of consciousness.[56][57]
The symptoms of ketotifen overdose may be described according to the affected system of the body. The cardiovascular effects of ketotifen overdose may include tachycardia, hypotension, arrhythmias, and cardiac arrest. The respiratory effects may include respiratory depression, sleep apnea, and pulmonary edema. The gastrointestinal effects may include nausea, vomiting, abdominal pain, diarrhea, and pancreatitis. The renal effects may include acute renal failure and urinary retention. The hepatic effects may include hepatitis and jaundice. The hematologic effects may include anemia, leukopenia, thrombocytopenia, and coagulopathy. The neurologic effects of ketotifen overdose may include convulsions, hyperexcitability, coma, and death. The risk of seizures is higher in children, especially those with a history of epilepsy or febrile seizures. The risk of coma and death is higher in adults, especially those with pre-existing medical conditions or concomitant use of other drugs that cause sedation or lower the seizure threshold.[55][56]
In children, ketotifen overdose may lead to toxic encephalopathy with lifelong health consequences. There was a reported case of an overdose in a 4-month-old boy that led to growth retardation and mental deterioration.[58][59][57]
Interactions
[edit]In systemic (oral) administration, ketotifen has the potential to enhance the effects of sedatives, hypnotics, antihistamines, and alcohol. Interactions have been observed between oral ketotifen and oral hypoglycemic agents, antihistamines, and medications with sedative properties.[60][61]
Oral ketotifen may interact with amphetamine and benzphetamine, which may decrease the activities of ketotifen.[62][63]
The concomitant use of oral ketotifen with amifampridine, bupropion, donepezil, and pitolisant is not recommended.[64]
In rare instances, patients who have been administered oral ketotifen with oral antidiabetic agents have exhibited a reversible decrease in thrombocyte count. As such, it is recommended to monitor thrombocyte counts in patients who are concurrently taking oral antidiabetic agents.[60][61]
Systemic use of ketotifen may decrease the effectiveness of benzylpenicilloyl polylysine as a diagnostic agent.[62] Ketotifen may affect the results of some laboratory tests, such as skin tests for allergies or blood glucose levels. Ketotifen may interfere with the skin test reactions by suppressing the histamine response, leading to false-negative results.[62]
Ophthalmic use of ketotifen may interact with contact lenses, as the eye drops may contain preservatives that can be absorbed by soft contact lenses and cause eye irritation.[65]
Pharmacology
[edit]Ketotifen is a selective antihistamine – that is, an inverse agonist of the histamine H1 receptor (Ki = 0.166 nM)[66] – and mast cell stabilizer.[67][68][69] By preventing the degranulation of mast cells, ketotifen inhibits the release of inflammatory mediators such as histamine and leukotrienes, which are implicated in allergic reactions.[67] Ketotifen action is also based on its inhibition of serotonin release.[67]
Ketotifen also plays a role in the prevention of accumulation of eosinophils, which are white blood cells that become active during allergic reactions and infections; as such, ketotifen helps in reducing inflammation this way.[67]
In addition, ketotifen has weak anticholinergic (Ki = 204 nM for mACh) and antiserotonergic (Ki = 38.9 nM for 5-HT2A) activity.[66][70] However, at the dosages in which it is typically used clinically, both the anticholinergic and antiserotonergic activity of ketotifen are said not to be appreciable.[71]
Ketotifen is a lipophilic compound that can cross the blood–brain barrier and exert central nervous system effects, such as sedation,[72] weight gain, and anticonvulsant activity. Ketotifen also has peripheral effects, such as inhibition of platelet aggregation, modulation of cytokine production, and enhancement of mucociliary clearance.[8][73][74]
Ketotifen acts as a mast cell stabilizer by preventing the degranulation and release of histamine and other inflammatory mediators, such as leukotrienes,[34] prostaglandins, and cytokines, from mast cells. Ketotifen also inhibits the activation and migration of eosinophils, basophils, and neutrophils, which are involved in the inflammatory response and tissue damage in allergic and respiratory diseases.[75][38][76]
Ketotifen has a dual mode of action as an antihistamine and a mast cell stabilizer, which makes it effective in the prophylaxis and treatment of various allergic and respiratory conditions, such as asthma, allergic rhinitis, conjunctivitis,[11] dermatitis, urticaria, and anaphylaxis. Ketotifen can also reduce the bronchial hyperreactivity and airway inflammation that are characteristic of chronic asthma.[77][14][76]
Ketotifen has a plasma half-life of about 12 hours. Ketotifen is extensively metabolized in the liver by oxidation and conjugation, and the metabolites are excreted in the urine and feces. The bioavailability of oral ketotifen is about 50% due to hepatic first-pass metabolism. Peak plasma concentration is reached in about 2 to 4 hours. The pharmacokinetics of ketotifen are not significantly affected by age, gender, or renal impairment, but may be altered by hepatic impairment or concomitant use of other drugs.[78]
Ketotifen, like other antihistamines,[72][79] is mainly metabolized by the cytochrome P450 (CYP) enzymes, especially CYP3A4[80][81] in the liver. The CYP enzymes are responsible for the oxidation and demethylation of ketotifen, producing the major metabolites norketotifen and 10-hydroxyketotifen. Norketotifen is pharmacologically active and has a similar potency as ketotifen, while 10-hydroxyketotifen is inactive. The metabolites are then conjugated with glucuronic acid or sulfate and excreted in the urine and feces.[82][83]
Classification
[edit]
Ketotifen is a noncompetitive H1-antihistamine and mast cell stabilizer.[85]
There is no academic consensus[8] on whether ketotifen should be classified as a medication belonging to the first[86][87][14] or the second generations of antihistamine drugs;[88][89] the classification can vary depending on the criteria used and the context of the study,[90] and is primarily based on chemical structure, pharmacological properties, and side effect profiles of an antihistamine drug.[91][90][8][92] First-generation H1 antihistamines, such as diphenhydramine, reduce skin reactivity for up to 24 hours, whereas ketotifen suppresses skin reactivity for over five days, a typical duration for the second generation of the class.[93] Ketotifen is a tricyclic, benzocycloheptene-based compound with chemical structures similar to first-generation antihistamines such as azatadine, cyproheptadine, chlorpheniramine, and diphenhydramine, and other compounds with antihistamine properties such as pizotifen. The sedative effects of ketotifen are also a reason for differences in classification. First-generation antihistamines are well known for their sedating side effects due to their ability to penetrate the blood–brain barrier.[91] While ketotifen has some sedative properties, it is generally considered to have a milder sedative effect compared to traditional first-generation antihistamines,[90][8] so this reduced sedation is one of the reasons why ketotifen is sometimes classified as a second-generation antihistamine.[92]
History
[edit]Ketotifen was patented in 1970 and came into medical use in 1976.[94] Ketotifen was developed and patented by Sandoz Pharmaceuticals (a part of Novartis), a Swiss company.[95][96][97]
Ketotifen was approved for medical use in Canada in December 1990.[2] Ketotifen was approved for medical use in the United States in July 1999.[98] TA contact lens with ketotifen was approved for medical use in the United States in 2022.[99][100]
Society and culture
[edit]
Brand names
[edit]Ketotifen is sold under various brand names worldwide, depending on country and formulation, with over 200 different names used.[101][102][103] In the United States, ketotifen fumarate ophthalmic solution is marketed under brand name Zaditor, which is owned by Alcon Inc., a Swiss-American pharmaceutical company.[104][105]
Litigation
[edit]There was a litigation related to ketotifen. In 2021, the plaintiff, Edward C. Hanks, brought an action in the United States District Court for the Central District of Illinois against the defendants, Ned Hubbard and others, alleging that they violated his rights under the Eighth Amendment to the United States Constitution by acting with deliberate indifference to his serious medical needs. The plaintiff claimed that he suffered from a chronic eye condition that required medical attention and that the defendant, Dr. Hubbard, prescribed him ketotifen. The plaintiff further claimed that the ketotifen eye drops caused him adverse reactions, such as severe pain, burning, and blurred vision, and that the defendant, Dr. Hubbard, failed to offer him an alternative medication or refer him to an ophthalmologist. The plaintiff also claimed that he sustained permanent eye damage as a result of the ketotifen. The district court granted the defendant's motion to dismiss, finding that the plaintiff failed to state a claim upon which relief could be granted. The plaintiff appealed to the United States Court of Appeals for the Seventh Circuit, which affirmed the district court's judgment on 7 February 2022.[106]
Research
[edit]Anatomy
[edit]Human mast cell heterogeneity (diversity) significantly impacts the efficacy of ketotifen in preventing mediator release (mast cell activation). In experiments, ketotifen inhibits mast cells from lung and tonsillar tissues when stimulated via an IgE-dependent histamine release mechanism. However, neither ketotifen nor disodium cromoglycate, another mast cell stabilizer, failed to inhibit mediator release from skin mast cells, that were unresponsive to these stabilizers. Such patterns of mast cell activation suggests the existence of different types of mast cells across various tissues—a topic of ongoing research.[107][108]
Metabolism
[edit]Research directions for ketotifen include the investigation of norketotifen (NK), a metabolite of ketotifen. In vitro studies using human liver microsomes and hepatocytes suggest that NK may be the major demethylated hepatic metabolite of ketotifen. Unlike ketotifen, NK does not seem to induce severe sedative effects, potentially allowing for higher doses to be administered without sedation as a limiting factor. Furthermore, NK may probably have potent and dose-dependent inhibition of the release of the pro-inflammatory cytokine TNF-α, suggesting potential anti-inflammatory activity. thus, ketotifen can probably be considered a sedating prodrug that converts to NK, a nonsedating metabolite with anti-inflammatory properties, when used as an anti-inflammatory medication.[109] The potential future applications of norketotifen are researched by Emergo Therapeutics, a US company.[110][111][112][113][114]
Conditions
[edit]Increased appetite and weight gain
[edit]The underlying mechanisms of why ketotifen (similarly to other antihistamine drugs such as astemizole, azelastine)[90] may increase appetite and lead to weight gain in some people, are not fully understood.[90]
Different studies have shown conflicting results about the amount of weight gain caused by ketotifen. In one study (postmarketing surveillance),[90] it was found that around 1 to 2 out of every 100 people who took the drug experienced weight gain, with adults gaining about 1 kilogram (2.2 lb) and children over the age of one gaining 2.8–3.3 kilograms (6.2–7.3 lb). However, in another study,[54] adults gained a higher amount of weight: 5.0–5.4 kilograms (11.0–11.9 lb).[54]
Ketotifen exhibits a chemical resemblance to pizotifen, a substance known for its appetite-stimulating properties.[90] One proposed mechanism of the increase in appetite involves the inhibitory effect of ketotifen on the production of TNF-α, which is a cytokine that plays a role in regulating energy metabolism. TNF-α can act directly on adipocytes (fat cells) to regulate the release of leptin. Leptin is a hormone produced by adipose tissue and acts as a satiety signal by binding to receptors in the hypothalamus, where it inhibits appetite. By reducing TNF-α production, ketotifen may lead to decreased leptin levels, reducing appetite control inhibition. Furthermore, ketotifen's influence on serotonin regulation could be involved in central serotonin disinhibition. Serotonin is known to have suppressant effects on appetite. It is suggested that ketotifen might cause a decrease in serotonin levels due to this regulatory influence. As a result, the decrease in serotonin function may lead to increased food intake tendencies and heightened appetite. Still, these potential mechanisms have been hypothesized based on limited evidence.[115] Studies on mice suggest that caffeine[115] or citrus aurantifolia oil[116] may prevent weight-gain induced by ketotifen, but, this has not been confirmed on human subjects.[116]
Irritable bowel syndrome
[edit]Ketotifen is being studied in context of a possible link between abnormalities in intestinal mast cells and irritable bowel syndrome, but there are no solid results yet.[108][117]
References
[edit]- ^ a b c d "Zaditor- ketotifen fumarate solution". DailyMed. 13 February 2020. Archived from the original on 11 June 2021. Retrieved 4 September 2020.
- ^ a b "Zaditen Product information". Health Canada. 22 October 2009. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ "Zaditor Product information". Health Canada. 22 October 2009. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ "Zaditen Summary of Product Characteristics (SmPC)". emc. 13 October 2020. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ a b c d e "Acuvue Theravision with ketotifen". DailyMed. 11 March 2022. Archived from the original on 3 December 2023.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Ketotifen Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Archived from the original on 4 June 2023. Retrieved 30 August 2024.
- ^ a b c d e f g Grant SM, Goa KL, Fitton A, Sorkin EM (September 1990). "Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders". Drugs. 40 (3): 412–448. doi:10.2165/00003495-199040030-00006. PMID 2226222. S2CID 242916740.
- ^ Ono J, Toshida H (July 2022). "Use of Ketotifen Fumarate-Eluting Daily Disposable Soft Contact Lens in Management of Ocular Allergy: Literature Review and Report of Two Cases". Cureus. 14 (7): e27093. doi:10.7759/cureus.27093. PMC 9391663. PMID 36000122.
- ^ García-Martín E, Canto G, Agúndez JA (November 2013). "Metabolic considerations of drugs in the treatment of allergic diseases". Expert Opin Drug Metab Toxicol. 9 (11): 1437–52. doi:10.1517/17425255.2013.823400. PMID 23902458. S2CID 30634949.
- ^ a b c d Dou XY, Zhang W (2023). "Topical ketotifen treatment for allergic conjunctivitis: a systematic review and Meta-analysis". Int J Ophthalmol. 16 (2): 286–292. doi:10.18240/ijo.2023.02.17. PMC 9922628. PMID 36816214.
- ^ a b c Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, et al. (October 2009). "EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria". Allergy. 64 (10): 1427–1443. doi:10.1111/j.1398-9995.2009.02178.x. PMID 19772513. S2CID 14587946.
- ^ Li Z, Celestin J (23 February 2015). Ketotifen: A Role in the Treatment of Idiopathic Anaphylaxis. American Academy of Allergy, Asthma & Immunology Annual Meeting. Houston.
- ^ a b c Sokol KC, Amar NK, Starkey J, Grant JA (December 2013). "Ketotifen in the management of chronic urticaria: resurrection of an old drug". Annals of Allergy, Asthma & Immunology. 111 (6): 433–436. doi:10.1016/j.anai.2013.10.003. PMC 4309375. PMID 24267353.
- ^ Shawky RM, Seifeldin NS (2015). "The relation between antihistamine medication during early pregnancy & birth defects". Egyptian Journal of Medical Human Genetics. 16 (4): 287–90. doi:10.1016/j.ejmhg.2015.04.003.
- ^ a b c Zuberbier T (January 2012). "A Summary of the New International EAACI/GA(2)LEN/EDF/WAO Guidelines in Urticaria". The World Allergy Organization Journal. 5 (Suppl 1): S1 – S5. doi:10.1186/1939-4551-5-S1-S1. PMC 3488932. PMID 23282889.
- ^ a b c d e Frieri M (June 2018). "Mast Cell Activation Syndrome". Clin Rev Allergy Immunol. 54 (3): 353–365. doi:10.1007/s12016-015-8487-6. PMID 25944644. S2CID 5723622.
- ^ Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. (2018). "The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets". Front Immunol. 9: 1873. doi:10.3389/fimmu.2018.01873. PMC 6099187. PMID 30150993.
- ^ Simons FE, Simons KJ (December 2011). "Histamine and H1-antihistamines: celebrating a century of progress". J Allergy Clin Immunol. 128 (6): 1139–1150.e4. doi:10.1016/j.jaci.2011.09.005. PMID 22035879.
- ^ Linton S, Hossenbaccus L, Ellis AK (October 2023). "Evidence-based use of antihistamines for treatment of allergic conditions". Ann Allergy Asthma Immunol. 131 (4): 412–420. doi:10.1016/j.anai.2023.07.019. PMID 37517656.
- ^ Ormerod AD (November 1994). "Urticaria. Recognition, causes and treatment". Drugs. 48 (5): 717–30. doi:10.2165/00003495-199448050-00006. PMID 7530629.
- ^ a b Sastre E, Caracuel L, Xavier FE, Balfagón G, Blanco-Rivero J (2013). "Opposite effect of mast cell stabilizers ketotifen and tranilast on the vasoconstrictor response to electrical field stimulation in rat mesenteric artery". PLOS ONE. 8 (8): e73232. Bibcode:2013PLoSO...873232S. doi:10.1371/journal.pone.0073232. PMC 3748149. PMID 23977380.
- ^ Suzuki Y, Inoue T, Ra C (14 February 2012). "Calcium Signaling in Mast Cells: Focusing on L-Type Calcium Channels". Calcium Signaling. Advances in Experimental Medicine and Biology. Vol. 740. pp. 955–977. doi:10.1007/978-94-007-2888-2_44. ISBN 978-94-007-2888-2. PMID 22453979.
- ^ Holowka D, Wilkes M, Stefan C, Baird B (April 2016). "Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress". Biochem Soc Trans. 44 (2): 505–9. doi:10.1042/BST20150273. PMC 5293407. PMID 27068962.
- ^ El-Alali EA, Abukhiran IM, Alhmoud TZ (July 2021). "Successful use of montelukast in eosinophilic gastroenteritis: a case report and a literature review". BMC Gastroenterology. 21 (1): 279. doi:10.1186/s12876-021-01854-x. PMC 8265096. PMID 34238222.
- ^ MacDonald G (1982). "An Overview of Ketotifen". Chest. 82 (1 Suppl): 30s – 32s. doi:10.1378/chest.82.1.30S. PMID 6806019. Archived from the original on 17 July 2024. Retrieved 17 July 2024.
- ^ Markham A, Goa KL (1996). "Ketotifen". Clinical Immunotherapeutics. 5 (5): 400–411. doi:10.1007/BF03259336. Archived from the original on 6 August 2024. Retrieved 17 July 2024.
- ^ "Acuvue Theravision with ketotifen (etafilcon A drug-eluting contact lens with ketotifen), for ophthalmic use" (PDF). 2022. Archived (PDF) from the original on 8 March 2024. Retrieved 13 April 2024.
- ^ a b c "Ketotifen ophthalmic Uses, Side Effects & Warnings". Archived from the original on 16 November 2023. Retrieved 16 November 2023.
- ^ "List of nationally authorised medicinal products" (PDF). European Medicines Agency. 14 June 2018. Archived (PDF) from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ "Ketotifen Update (4th December 2019)". 4 December 2019. Archived from the original on 29 November 2023. Retrieved 13 April 2024.
- ^ Schwarzer G, Bassler D, Mitra A, Ducharme FM, Forster J (2004). "Ketotifen alone or as additional medication for long-term control of asthma and wheeze in children". Cochrane Database Syst Rev. 2004 (1): CD001384. doi:10.1002/14651858.CD001384.pub2. PMC 8406918. PMID 14973969.
- ^ Grahnén A, Lönnebo A, Beck O, Eckernäs SA, Dahlström B, Lindström B (May 1992). "Pharmacokinetics of ketotifen after oral administration to healthy male subjects". Biopharmaceutics & Drug Disposition. 13 (4): 255–262. doi:10.1002/bdd.2510130404. PMID 1600111. S2CID 72293850.
- ^ a b Zhu TH, Zou G, Ding SJ, Li TT, Zhu LB, Wang JZ, et al. (2019). "Mast cell stabilizer ketotifen reduces hyperalgesia in a rodent model of surgically induced endometriosis". J Pain Res. 12: 1359–1369. doi:10.2147/JPR.S195909. PMC 6500880. PMID 31118754.
Ketotifen has a stronger effect on stabilizing MCs than sodium cromoglycate. This drug has antihistamine activity and is also a functional leukotriene antagonist
- ^ Bäck M (2016). "Leukotrienes". Compendium of Inflammatory Diseases. pp. 849–857. doi:10.1007/978-3-7643-8550-7_105. ISBN 978-3-7643-8530-9.
- ^ Sasaki F, Yokomizo T (August 2019). "The leukotriene receptors as therapeutic targets of inflammatory diseases". Int Immunol. 31 (9): 607–615. doi:10.1093/intimm/dxz044. PMID 31135881.
- ^ Mostafa GA, Bakheit A, AlMasoud N, AlRabiah H (April 2021). "Charge Transfer Complexes of Ketotifen with 2,3-Dichloro-5,6-dicyano-p-benzoquinone and 7,7,8,8-Tetracyanoquodimethane: Spectroscopic Characterization Studies". Molecules. 26 (7): 2039. doi:10.3390/molecules26072039. PMC 8038309. PMID 33918481.
- ^ a b Castillo JG, Gamboa PM, García BE, Oehling A (1990). "Effect of ketotifen on phosphodiesterase activity from asthmatic individuals". Allergologia et Immunopathologia. 18 (4): 197–201. PMID 1702263.
- ^ Omori K, Kotera J (February 2007). "Overview of PDEs and their regulation". Circ Res. 100 (3): 309–27. doi:10.1161/01.RES.0000256354.95791.f1. PMID 17307970.
- ^ Feneck R (1 December 2007). "Phosphodiesterase inhibitors and the cardiovascular system". Continuing Education in Anaesthesia Critical Care & Pain. 7 (6): 203–207. doi:10.1093/bjaceaccp/mkm039.
- ^ a b c "Ketotifen Monograph for Professionals". Archived from the original on 11 June 2021. Retrieved 16 November 2023.
- ^ "Ketotifen - Mechanism, Indication, Contraindications, Dosing, Adverse Effect, Interaction, Hepatic Dose; Drug Index; Pediatric Oncall". Pediatric Oncall. Archived from the original on 16 November 2023. Retrieved 16 November 2023.
- ^ Baselga E, Torrelo A (2008). "Inflammatory and Purpuric Eruptions". Neonatal Dermatology. pp. 311–342. doi:10.1016/B978-1-4160-3432-2.50022-4. ISBN 978-1-4160-3432-2.
- ^ Chaplin S, Scadding G (2011). "Antihistamines: Their properties and use in hay fever". Prescriber. 22 (10): 29–31. doi:10.1002/psb.758.
Some antihistamines should not be used in people with acute porphyria. Those believed to be safe are chlorphenamine, desloratadine, fexofenadine, ketotifen (Zaditen), loratadine and promethazine (Phenergan)
- ^ Yokoyama H, Iinuma K (18 November 2012) [May 1996]. "Histamine and Seizures". CNS Drugs. 5 (5): 321–330. doi:10.2165/00023210-199605050-00002. PMID 26071045.
- ^ "Ketotifen (Oral) Advanced Patient Information". Archived from the original on 2 December 2023. Retrieved 11 March 2024.
- ^ Waterfield J (29 September 2013). "Antihistamines: Mode of action, prescribing rationale and uses". Nurse Prescribing. 7 (4): 166–170. doi:10.12968/npre.2009.7.4.41711.
- ^ Chaplin S, Scadding G (13 June 2011). "Antihistamines: Their properties and use in hay fever". Prescriber. 22 (10): 29–31. doi:10.1002/psb.758.
- ^ Jue TL, Storm AC, Naveed M, Fishman DS, Qumseya BJ, McRee AJ, et al. (2021). "ASGE guideline on the role of endoscopy in the management of benign and malignant gastroduodenal obstruction". Gastrointestinal Endoscopy. 93 (2): 309–322.e4. doi:10.1016/j.gie.2020.07.063. PMID 33168194.
- ^ Shim K (2019). "Endoscopic Management for Pyloric Stricture and Gastric Outlet Obstruction: Dilation and Stenting". Therapeutic Gastrointestinal Endoscopy. pp. 277–289. doi:10.1007/978-981-13-1184-0_16. ISBN 978-981-13-1184-0.
- ^ Bielory L (2002). "Update on ocular allergy treatment". Expert Opinion on Pharmacotherapy. 3 (5): 541–553. doi:10.1517/14656566.3.5.541. PMID 11996633.
- ^ "Angle-Closure Glaucoma - Angle-Closure Glaucoma". Archived from the original on 17 May 2024. Retrieved 15 October 2024.
- ^ a b Ketotifen. National Institute of Child Health and Human Development. 2006. PMID 30000587. NCBI NBK501527. Archived from the original on 3 October 2023. Retrieved 22 November 2023.
- ^ a b c "Zaditen - MIMS online". www.mims.co.uk. Archived from the original on 25 October 2020. Retrieved 2 August 2017.
- ^ a b c Le Blaye I, Donatini B, Hall M, Krupp P (1992). "Acute ketotifen overdosage. A review of present clinical experience". Drug Safety. 7 (5): 387–392. doi:10.2165/00002018-199207050-00007. PMID 1418695. S2CID 25839342.
- ^ a b c d Jeffreys DB, Volans GN (May 1981). "Ketotifen overdose: surveillance of the toxicity of a new drug". British Medical Journal. 282 (6278): 1755–1756. doi:10.1136/bmj.282.6278.1755. PMC 1505736. PMID 6113023.
- ^ a b c d "ACE inhibitors". Reactions Weekly (422): 5. 1992. doi:10.2165/00128415-199204220-00009.
- ^ Yokoyama H, Hirose M, Uematsu M, Haginoya K, Iinuma K, Kimura S (2012). "Ketotifen overdose. Toxic encephalopathy, epilepsy and mental retardation in an infant: case report". Pediatrics International. 54 (6): 963. doi:10.1111/j.1442-200X.2012.03718.x. PMID 23279031. Archived from the original on 13 April 2024. Retrieved 3 April 2024.
In the present case, a 4-month-old boy was administered ketotifen at 5 times the recommended dose, and he showed mental deterioration and growth retardation. The presence of developmental deterioration strongly suggests that overdose of ketotifen induces toxic encephalopathy.
- ^ "Ketotifen overdose". Reactions Weekly. 1447: 25. 2013. doi:10.1007/s40278-013-2459-5.
- ^ a b "Ketotifen 2mg Tablets, 1mg/5mL Oral Solution (product monograph)" (PDF). Archived (PDF) from the original on 18 July 2022. Retrieved 11 March 2024.
- ^ a b "Tablets, 1 mg ketotifen (as ketotifen hydrogen fumarate), Oral (product monograph)" (PDF). Archived (PDF) from the original on 21 February 2024. Retrieved 11 March 2024.
- ^ a b c Greenwood C (July 1982). "The pharmacology of ketotifen". Chest. 82 (1 Suppl): 45S – 48S. doi:10.1378/chest.82.1_supplement.45s (inactive 3 December 2024). PMID 6123414.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ Rogóz Z, Skuza G, Sowińska H (1981). "Central action of ketotifen". Polish Journal of Pharmacology and Pharmacy. 33 (5): 503–515. PMID 7335554.
- ^ "Ketotifen (Oral) Advanced Patient Information". Archived from the original on 2 December 2023. Retrieved 11 March 2024.
- ^ "Ketotifen Ophthalmic: Generic, Uses, Side Effects, Dosages, Interactions, Warnings". Archived from the original on 24 February 2024. Retrieved 1 December 2023.
- ^ a b Kakiuchi M, Ohashi T, Musoh K, Kawamura K, Morikawa K, Kato H (April 1997). "Studies on the novel antiallergic agent HSR-609: its penetration into the central nervous system in mice and guinea pigs and its selectivity for the histamine H1-receptor". Japanese Journal of Pharmacology. 73 (4): 291–298. doi:10.1254/jjp.73.291. PMID 9165365.
- ^ a b c d Ma C, Li H, Lu S, Li X, Wang S, Wang W (2023). "Tryptase and Exogenous Trypsin: Mechanisms and Ophthalmic Applications". J Inflamm Res. 16: 927–939. doi:10.2147/JIR.S402900. PMC 9987324. PMID 36891173.
- ^ Nelson WL (2008). "Antihistamines and Related Antiallergic and Antiulcer Agents". In Lemke TL, Williams DA (eds.). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1019–. ISBN 978-0-7817-6879-5.
- ^ Ang DC, Hilligoss J, Stump T (September 2015). "Mast Cell Stabilizer (Ketotifen) in Fibromyalgia: Phase 1 Randomized Controlled Clinical Trial". The Clinical Journal of Pain. 31 (9): 836–842. doi:10.1097/AJP.0000000000000169. PMC 4417653. PMID 25370135.
- ^ Alagarsamy V (16 June 2012). "Antihistamines". Textbook of Medicinal Chemistry Vol II - E-Book. Elsevier Health Sciences. pp. 38–. ISBN 978-81-312-3259-0.
- ^ Drews J (6 December 2012). "Substances with an Antialergic Effect". Immunopharmacology: Principles and Perspectives. Springer Science & Business Media. pp. 282–. ISBN 978-3-642-75561-3.
- ^ a b Li L, Liu R, Peng C, Chen X, Li J (July 2022). "Pharmacogenomics for the efficacy and side effects of antihistamines". Exp Dermatol. 31 (7): 993–1004. doi:10.1111/exd.14602. PMID 35538735.
- ^ Muñoz-Cano RM, Casas-Saucedo R, Valero Santiago A, Bobolea I, Ribó P, Mullol J (August 2019). "Platelet-Activating Factor (PAF) in Allergic Rhinitis: Clinical and Therapeutic Implications". J Clin Med. 8 (9): 1338. doi:10.3390/jcm8091338. PMC 6780525. PMID 31470575.
- ^ Kahhak L, Roche A, Dubray C, Arnoux C, Benveniste J (May 1996). "Decrease of ciliary beat frequency by platelet activating factor: protective effect of ketotifen". Inflamm Res. 45 (5): 234–8. doi:10.1007/BF02259609. PMID 8737746.
- ^ Luna-Gomes T, Bozza PT, Bandeira-Melo C (2013). "Eosinophil recruitment and activation: the role of lipid mediators". Front Pharmacol. 4: 27. doi:10.3389/fphar.2013.00027. PMC 3605515. PMID 23525348.
- ^ a b Martín AP, Urrets-Zavalia J, Berra A, Mariani AL, Gallino N, Gomez Demel E, et al. (January 2003). "The effect of ketotifen on inflammatory markers in allergic conjunctivitis: an open, uncontrolled study". BMC Ophthalmol. 3: 2. doi:10.1186/1471-2415-3-2. PMC 140320. PMID 12515585.
- ^ Stone M, Francisco JC, Kumar NN, Barboza J (1 January 2014). "Oral Mast Cell Stabilizers". Encyclopedia of Medical Immunology. pp. 551–555. doi:10.1007/978-1-4614-9194-1_242. ISBN 978-1-4614-9193-4.
- ^ Fahmy RH, Badr-Eldin SM (August 2014). "Novel delivery approach for ketotifen fumarate: dissofilms formulation using 3² experimental design: in vitro/in vivo evaluation". Pharm Dev Technol. 19 (5): 521–30. doi:10.3109/10837450.2013.800108. PMID 23713715. S2CID 45012360.
- ^ Merk HF (November 2001). "Standard treatment: the role of antihistamines". J Investig Dermatol Symp Proc. 6 (2): 153–6. doi:10.1046/j.0022-202x.2001.00032.x. PMID 11764306.
- ^ El-Kommos ME, El-Gizawy SM, Atia NN, Hosny NM (2015). "Analysis for commonly prescribed non-sedating antihistamines". Analytical Chemistry Research. 3: 1–12. doi:10.1016/j.ancr.2014.11.003.
- ^ Jáuregui I, Mullol J, Bartra J, del Cuvillo A, Dávila I, Montoro J, et al. (2006). "H1 antihistamines: psychomotor performance and driving". J Investig Allergol Clin Immunol. 16 (Suppl 1): 37–44. PMID 17357376.
- ^ Lieberman P, Hernandez-Trujillo V, Lieberman J, Frew AJ (2008). "Antihistamines". Clinical Immunology. pp. 1317–1329. doi:10.1016/B978-0-323-04404-2.10089-2. ISBN 978-0-323-04404-2. Archived from the original on 24 February 2024. Retrieved 14 February 2024.
- ^ "Center for drug evaluation and research. Application no. 21-066" (PDF). Archived (PDF) from the original on 14 February 2024. Retrieved 14 February 2024.
- ^ Eltze M, Mutschler E, Lambrecht G (1992). "Affinity profiles of pizotifen, ketotifen and other tricyclic antimuscarinics at muscarinic receptor subtypes M1, M2 and M3". European Journal of Pharmacology. 211 (3): 283–293. doi:10.1016/0014-2999(92)90383-F. PMID 1377628.
- ^ Sarcina D, Giovannini M, Oranges T, Barni S, Pedaci FA, Liccioli G, et al. (2021). "Case Report and Review of the Literature: Bullous Skin Eruption After the Booster-Dose of Influenza Vaccine in a Pediatric Patient With Polymorphic Maculopapular Cutaneous Mastocytosis". Front Immunol. 12. doi:10.3389/fimmu.2021.688364. PMC 8322976. PMID 34335590.
- ^ Bittner L, Teixidó E, Keddi I, Escher BI, Klüver N (May 2019). "pH-Dependent Uptake and Sublethal Effects of Antihistamines in Zebrafish (Danio rerio) Embryos". Environmental Toxicology and Chemistry. 38 (5): 1012–1022. Bibcode:2019EnvTC..38.1012B. doi:10.1002/etc.4395. PMID 30779379. S2CID 73482611.
- ^ Pinke KH, Zorzella-Pezavento SF, de Campos Fraga-Silva TF, Mimura LA, de Oliveira LR, Ishikawa LL, et al. (January 2020). "Calming Down Mast Cells with Ketotifen: A Potential Strategy for Multiple Sclerosis Therapy?". Neurotherapeutics. 17 (1): 218–234. doi:10.1007/s13311-019-00775-8. PMC 7007452. PMID 31463682.
- ^ Janeczko P, Norris MR, Bielory L (October 2021). "Assessment of receptor affinities of ophthalmic and systemic agents in dry eye disease". Current Opinion in Allergy and Clinical Immunology. 21 (5): 480–485. doi:10.1097/ACI.0000000000000773. PMID 34387278. S2CID 236998913.
- ^ Triantafillou V, Maina IW, Patel NN, Tong CC, Papagiannopoulos P, Kohanski MA, et al. (February 2020). "In vitro safety of ketotifen as a topical nasal rinse". International Forum of Allergy & Rhinology. 10 (2): 265–270. doi:10.1002/alr.22461. PMID 32086998. S2CID 211246051.
- ^ a b c d e f g Slater JW, Zechnich AD, Haxby DG (January 1999). "Second-generation antihistamines: a comparative review". Drugs. 57 (1): 31–47. doi:10.2165/00003495-199957010-00004. PMID 9951950. S2CID 24659435.
- ^ a b Sagara A, Nagahama A, Aki H, Yoshimura H, Hiraide M, Shimizu T, et al. (October 2023). "Potential risk of driving performance under combined conditions of taking second-generation antihistamines and attending calls using a hands-free function". Traffic Injury Prevention. 25 (1): 36–40. doi:10.1080/15389588.2023.2265002. PMID 37815801. S2CID 263801715.
- ^ a b Aelony Y (September 1998). "First-generation vs second-generation antihistamines". Archives of Internal Medicine. 158 (17): 1949–1950. doi:10.1001/archinte.158.17.1949 (inactive 24 November 2024). PMID 9759694.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Chiriac AM, Bousquet J, Demoly P (2017). "Principles of Allergy Diagnosis". Middleton's Allergy Essentials. pp. 117–131. doi:10.1016/B978-0-323-37579-5.00005-2. ISBN 978-0-323-37579-5. Archived from the original on 24 February 2024. Retrieved 14 February 2024.
- ^ Alapi EM, Fischer J (2006). "Table of Selected Analogue Classes". In Fischer J, Ganellin CR (eds.). Analogue-based Drug Discovery. John Wiley & Sons. p. 548. ISBN 978-3-527-60749-5. Archived from the original on 10 January 2023. Retrieved 1 August 2020.
- ^ "Ketotifen". Drugbank. 12 April 2024. Archived from the original on 6 August 2019. Retrieved 16 November 2023.
- ^ "Ketotifen Fumarate". Inxight Drugs. Bethesda MD, US: National Center for Advancing Translational Sciences (NCATS). 12 April 2024. Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ MacDonald G (1982). "An Overview of Ketotifen". Chest. 82 (1 Suppl): 30s – 32s. doi:10.1378/chest.82.1.30S. PMID 6806019. Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ "Drug Approval Package: Zaditor (Ketotifen Fumarate) NDA# 21-066". accessdata.fda.gov. 20 November 2001. Archived from the original on 8 March 2024. Retrieved 10 March 2024.
- ^ "Drug Approval Package: Acuvue Theravision with ketotifen". accessdata.fda.gov. 19 September 2022. Archived from the original on 10 March 2024. Retrieved 10 March 2024.
- ^ "Johnson & Johnson Vision Care Receives FDA Approval for Acuvue Theravision with Ketotifen – World's First and Only Drug-Eluting Contact Lens". Johnson and Johnson Vision (Press release). 2 March 2022. Archived from the original on 11 March 2024. Retrieved 10 March 2024.
- ^ "Ketotifen International". Drugs.com. Archived from the original on 11 April 2021. Retrieved 4 September 2020.
- ^ Tata (28 March 2024). "What To Know About Ketotifen (popular Brands: Ketasma And Asthafen)". MSN. Archived from the original on 13 April 2024. Retrieved 2 April 2024.
- ^ "Ketotifen (Ingredient)". Archived from the original on 2 April 2024. Retrieved 2 April 2024.
- ^ "DailyMed - ZADITOR- ketotifen fumarate solution". National Institutes of Health. 15 December 2023. Archived from the original on 11 June 2021. Retrieved 5 September 2020.
- ^ "Trademark search - ZADITOR". USPTO. 8 April 2024. Archived from the original on 13 July 2021. Retrieved 8 April 2024.
- ^ Hanks v. Hubbard (C.D. Ill 8 March 2021).
- ^ Finn DF, Walsh JJ (September 2013). "Twenty-first century mast cell stabilizers". Br J Pharmacol. 170 (1): 23–37. doi:10.1111/bph.12138. PMC 3764846. PMID 23441583.
- ^ a b Zhang L, Song J, Hou X (April 2016). "Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside". Journal of Neurogastroenterology and Motility. 22 (2): 181–192. doi:10.5056/jnm15137. PMC 4819856. PMID 26755686.
- ^ Aberg AK, Arulnesan N, Bolger GT, Ciofalo VB, Pucaj K, Walle K, et al. (April 2022). "Ketotifen is a Prodrug. Norketotifen is the active metabolite". Drug Development Research. 83 (2): 362–367. doi:10.1002/ddr.21865. PMID 34410005. S2CID 237216445.
- ^ "A Phase 2b Double-blind, Randomized, Placebo-controlled, Parallel-group Study of the Efficacy and Safety of Norketotifen (NKT) in the Treatment of Acute Uncomplicated Influenza-like Illness (ILI)". 25 January 2023. Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ "Efficacy and Safety of Norketotifen in Uncomplicated Influenza-like Illness: Influenza Clinical". 30 January 2023. Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ "Efficacy and Safety of Norketotifen in Uncomplicated Influenza-like Illness". 25 January 2023. Archived from the original on 20 April 2024. Retrieved 13 April 2024.
- ^ "Emergo finds midstage success in developing flu-fighter norketotifen + | Bioworld | BioWorld". Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ "Norketotifen in Influenza -Like Illness - Clinical Trials Registry - ICH GCP". Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ^ a b Habibi Asl B, Vaez H, Imankhah T, Hamidi S (2014). "Impact of caffeine on weight changes due to ketotifen administration". Advanced Pharmaceutical Bulletin. 4 (1): 83–89. doi:10.5681/apb.2014.013. PMC 3885374. PMID 24409414.
- ^ a b Asnaashari S, Delazar A, Habibi B, Vasfi R, Nahar L, Hamedeyazdan S, et al. (December 2010). "Essential oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice". Phytotherapy Research. 24 (12): 1893–1897. doi:10.1002/ptr.3227. PMID 20623616. S2CID 8888404. Archived from the original on 28 January 2024. Retrieved 10 January 2024.
- ^ Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, et al. (September 2010). "The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome" (PDF). Gut. 59 (9): 1213–1221. doi:10.1136/gut.2010.213108. PMID 20650926. S2CID 18889707. Archived (PDF) from the original on 11 June 2021. Retrieved 24 September 2019.
