User:Berkeleyone/sandbox
Tsuji-Wacker Oxidation
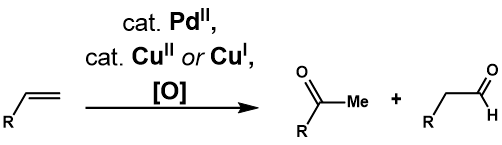
[edit]The advent of Wacker Process has spurred on many investigations into the utility and applicability of the reactions to more complex terminal olefins. The Tsuji-Wacker Oxidation is the palladium(II)-catalyzed transformation of such olefins into carbonyl compounds. Clement and Selwitz[1] were the first to find that using an aqueous DMF as solvent allowed for the oxidation of of 1-dodecene to 2-dodecanone, which addressed the insolubility problem of higher order olefins in water. Fahey[2] noted the use of 3-methylsulfolane in place of DMF as solvent increased the yield of oxidation of 3,3-Dimethylbut-1-ene. Two years after, Tsuji[3] applied the Selwitz conditions for selective oxidations of terminal olefins with multiple functional groups, and demonstrated its utility in synthesis of complex substrates[4]. Further development of the reaction has led to various catalytic systems to address selectivity of the reaction, as well as introduction of intermolecular and intramolecular oxidations with non-water nucleophiles.
Regioselectivity
[edit]Markovnikov Addition
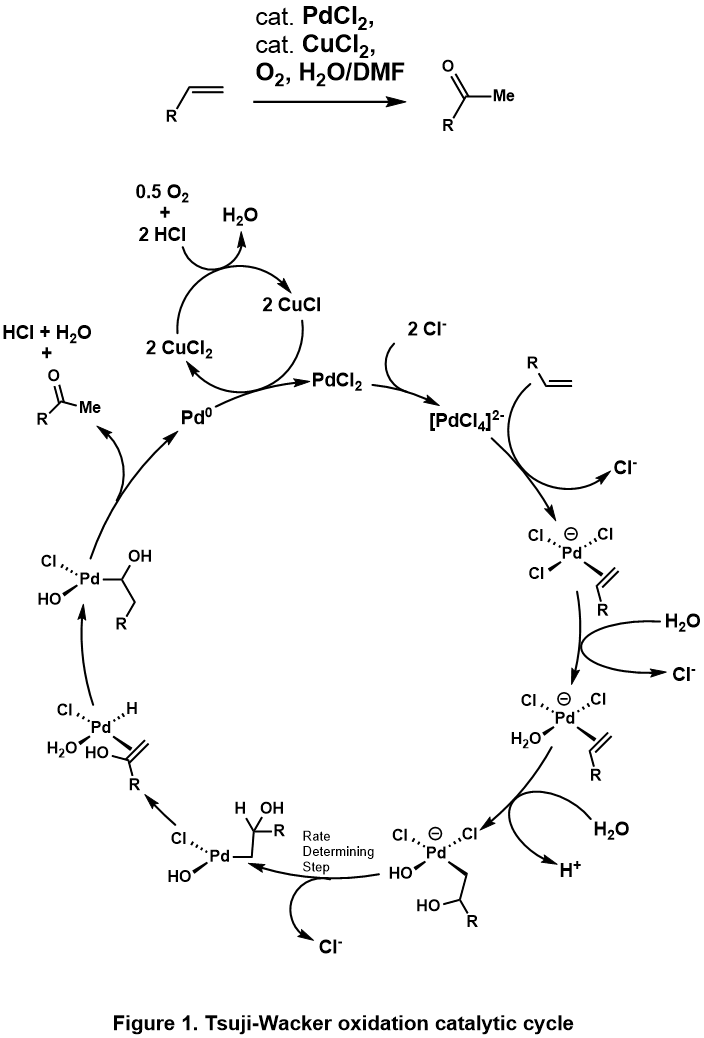
[edit]The Tsuji-Wacker Oxidation oxidizes terminal olefin to the corresponding methyl ketone under the Wacker process condition. Almost identical to that of Wacker Process, the proposed catalytic cycle[5](Figure 1) begins with complexation of PdCl2 and two chloride anions to PdCl4, which then undergoes subsequent ligand exchange of two chloride ligand for water and alkene to form Pd(Cl2)(H2O)(alkene) complex. A water molecule then attacks the olefin regioselectively through an outer sphere mechanism in a Markovnikov fashion, to form the more thermodynamically stable Pd(Cl2)(OH)(-CH2-CHOH-R) complex. Dissociation of a chloride ligand to the three coordinate palladium complex promotes β-hydride elimination, then subsequent 1,2-hydride insertion generates Pd(Cl2)(OH)(-CHOHR-CH3) complex. This undergoes β-hydride elimination to release the ketone, and subsequent reductive elimination produces HCl, water, and palladium(0). Finally palladium(0) is reoxidized to PdCl2 with two equivalents of Cu(II)Cl2, which in turn can be reoxidized by O2.
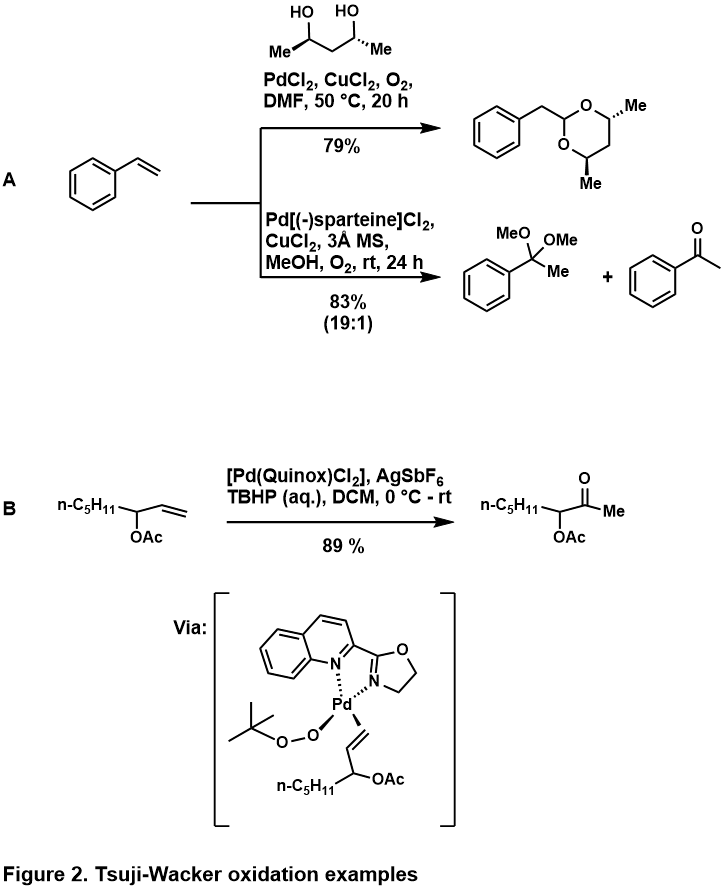
The oxidation of terminal olefins generally provide the Markovnikov ketone product, however in cases where substrate favors the aldehyde (discussed below) , different ligands can be used to enforce the Markovnikov regioselectivity. The use of sparteine as a ligand (Figure 2, A)[6] favors nucleopalladation at the terminal carbon to minimize steric interaction between the palladium complex and substrate. The Quinox-ligated palladium catalyst is used to favor ketone formation when substrate contains a directing group (Figure 2, B)[7]. When such substrate bind to Pd(Quinox)(OOtBu), this complex is coordinately saturated which prevents the binding of the directing group, and results in formation of the Markovnikov product. The efficiency of this ligand is also attributed to its electronic property, where anionic TBHP prefers to bind trans to the oxazoline and olefin coordinate trans to the quinoline[8].
Anti-Markovnikov Addition
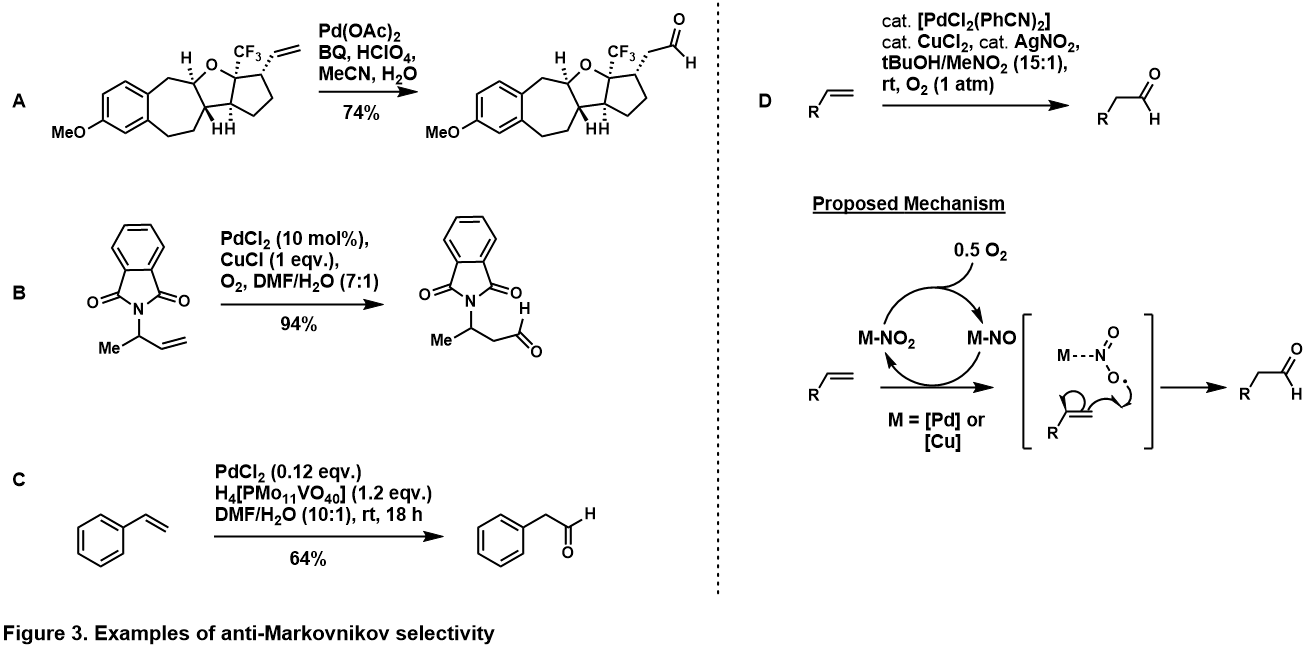
[edit]The anti-Markovnikov selectivity to aldehyde can be achieved through exploiting inherent stereoelectronics of the substrate[9]. Placement of directing group at homo-allylic (i.e. Figure 3, A)[10] and allylic position (i.e. Figure 3, B)[11] to the terminal olefin favors the anti-Markovnikov aldehyde product, which suggests that in the catalytic cycle the directing group chelates to the palladium complex such that water attacks at the anti-Markovnikov carbon to generate the more thermodynamically stable palladocycle. Anti-Markovnikov selectivity is also observed in styrenyl substrates (i.e. Figure 3, C)[12], presumably via η4-palladium-styrene complex after water attacks anti-Markovnikov. More examples of substrate-controlled, anti-Markovnikov Tsuji-Wacker Oxidation of olefins are given in reviews by Namboothiri[13], Feringa[14], and Muzart[15].
Grubbs and co-workers paved way for anti-Markovnikov oxidation of stereoelectronically unbiased terminal olefins, through the use of palladium-nitrite system (Figure 2, D)[16]. In his system, the terminal olefin was oxidized to the aldehyde with high selectivity through a catalyst-control pathway. The mechanism is under investigation, however evidence[13] suggests it goes through a nitrite radical adds into the terminal carbon to generate the more thermodynamically stable, secondary radical. Grubbs expanded this methodology to more complex, unbiased olefins[17][18].
Scope
[edit]Oxygen Nucleophiles
[edit]The intermolecular oxidations of olefins with alcohols as nucleophile typically generate ketals, where as the palladium-catalyzed oxidations of olefins with carboxylic acids as nucleophile genreats vinylic or allylic carboxylates. In case of diols, their reactions with alkenes typically generate ketals, whereas reactions of olefins bearing electron-withdrawing groups tend to form acetals[19].
Palladium-catalyzed intermolecular oxidations of dienes with carboxylic acids and alcohols as donors give 1,4-addition products. In the case of cyclohexadiene (Figure 4, A), Backvall found that stereochemical outcome of product was found to depend on concentration of LiCl[20]. This reaction proceeds by first generating the Pd(OAc)(benzoquinone)(allyl) complex, through anti-nucleopalladation of diene with acetate as nucleophile. The absence of LiCl induces an inner sphere reductive elimination to afford the trans-acetate stereochemistry to give the trans-1,4-adduct. The presence of LiCl displaces acetate with chloride due to its higher binding affinity, which forces an outer sphere acetate attack anti to the palladium, and affords the cis-acetate stereochemistry to give the cis-1,4-adduct. Intramolecular oxidative cyclization: 2-(2-cyclohexenyl)phenol cyclizes to corresponding dihydro-benzofuran (Figure 4, B)[21]; 1-cyclohexadiene-acetic acid in presence of acetic acid cyclizes to corresponding lactone-acetate 1,4 adduct (Figure 4, C)[22], with cis and trans selectivity controlled by LiCl presence.

Nitrogen Nucleophiles
[edit]The oxidative aminations of olefins are generally conducted with amides or imides; amines are thought to be protonated by the acidic medium or to bind the metal center too tightly to allow for the catalytic chemistry to occur[19]. These nitrogen nucleophiles are found to be competent in both intermolecular and intramolecular reactions, some examples are depicted (Figure 5, A[23], B[24])

References
[edit]- ^ Clement, William H.; Selwitz, Charles M. (1964-1). "Improved Procedures for Converting Higher α-Olefins to Methyl Ketones with Palladium Chloride". The Journal of Organic Chemistry. 29 (1): 241–243. doi:10.1021/jo01024a517. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Fahey, Darryl R.; Zeuch, Ernest A. (1974-11). "Aqueous sulfolane as solvent for rapid oxidation of higher .alpha.-olefins to ketones using palladium chloride". The Journal of Organic Chemistry. 39 (22): 3276–3277. doi:10.1021/jo00936a023. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Tsuji, Jiro; Shimizu, Isao; Yamamoto, Keiji (1976-08). "Convenient general synthetic method for 1,4- and 1,5-diketones by palladium catalyzed oxidation of α-allyl and α-3-butenyl ketones". Tetrahedron Letters. 17 (34): 2975–2976. doi:10.1016/s0040-4039(01)85504-0. ISSN 0040-4039.
{{cite journal}}: Check date values in:|date=(help) - ^ Tsuji, Jiro (1984). "Synthetic Applications of the Palladium-Catalyzed Oxidation of Olefins to Ketones". Synthesis. 1984 (05): 369–384. doi:10.1055/s-1984-30848. ISSN 0039-7881.
- ^ Kurti, Laszlo; Czako, Barbara (2005). Strategic Applications of named Reactions in Organic Synthesis. 525 B Street, Suite 1900, San Diego, California 92101-4495, USA: Elsevier Academic Press. p. 474. ISBN 0-12-429785-4.
{{cite book}}: CS1 maint: location (link) - ^ Balija, Amy M.; Stowers, Kara J.; Schultz, Mitchell J.; Sigman, Matthew S. (2006-3). "Pd(II)-Catalyzed Conversion of Styrene Derivatives to Acetals: Impact of (−)-Sparteine on Regioselectivity". Organic Letters. 8 (6): 1121–1124. doi:10.1021/ol053110p. ISSN 1523-7060.
{{cite journal}}: Check date values in:|date=(help) - ^ Michel, Brian W.; Camelio, Andrew M.; Cornell, Candace N.; Sigman, Matthew S. (2009-05-06). "A General and Efficient Catalyst System for a Wacker-Type Oxidation Using TBHP as the Terminal Oxidant: Application to Classically Challenging Substrates". Journal of the American Chemical Society. 131 (17): 6076–6077. doi:10.1021/ja901212h. ISSN 0002-7863. PMC 2763354. PMID 19364100.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Michel, Brian W.; Steffens, Laura D.; Sigman, Matthew S. (2011-6). "On the Mechanism of the Palladium-Catalyzed tert -Butylhydroperoxide-Mediated Wacker-Type Oxidation of Alkenes Using Quinoline-2-Oxazoline Ligands". Journal of the American Chemical Society. 133 (21): 8317–8325. doi:10.1021/ja2017043. ISSN 0002-7863. PMC 3113657. PMID 21553838.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Dong, Jia Jia; Browne, Wesley R.; Feringa, Ben L. (2014-11-03). "Palladium-Catalyzed anti-Markovnikov Oxidation of Terminal Alkenes". Angewandte Chemie International Edition. 54 (3): 734–744. doi:10.1002/anie.201404856. ISSN 1433-7851.
- ^ Miller, D. G.; Wayner, Danial D. M. (1990-04). "Improved method for the Wacker oxidation of cyclic and internal olefins". The Journal of Organic Chemistry. 55 (9): 2924–2927. doi:10.1021/jo00296a067. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Stragies, Roland; Blechert, Siegfried (2000-10). "Enantioselective Synthesis of Tetraponerines by Pd- and Ru-Catalyzed Domino Reactions". Journal of the American Chemical Society. 122 (40): 9584–9591. doi:10.1021/ja001688i. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Wright, Joseph A.; Gaunt, Matthew J.; Spencer, Jonathan B. (2006-01-11). "Novel Anti-Markovnikov Regioselectivity in the Wacker Reaction of Styrenes". Chemistry - A European Journal. 12 (3): 949–955. doi:10.1002/chem.200400644. ISSN 0947-6539.
- ^ a b Baiju, Thekke Veettil; Gravel, Edmond; Doris, Eric; Namboothiri, Irishi N.N. (2016-09). "Recent developments in Tsuji-Wacker oxidation". Tetrahedron Letters. 57 (36): 3993–4000. doi:10.1016/j.tetlet.2016.07.081. ISSN 0040-4039.
{{cite journal}}: Check date values in:|date=(help) - ^ Dong, Jia Jia; Browne, Wesley R.; Feringa, Ben L. (2014-11-03). "Palladium-Catalyzed anti-Markovnikov Oxidation of Terminal Alkenes". Angewandte Chemie International Edition. 54 (3): 734–744. doi:10.1002/anie.201404856. ISSN 1433-7851.
- ^ Muzart, Jacques (2007-08). "Aldehydes from Pd-catalysed oxidation of terminal olefins". Tetrahedron. 63 (32): 7505–7521. doi:10.1016/j.tet.2007.04.001. ISSN 0040-4020.
{{cite journal}}: Check date values in:|date=(help) - ^ Wickens, Zachary K.; Morandi, Bill; Grubbs, Robert H. (2013-09-13). "Aldehyde-Selective Wacker-Type Oxidation of Unbiased Alkenes Enabled by a Nitrite Co-Catalyst". Angewandte Chemie International Edition. 52 (43): 11257–11260. doi:10.1002/anie.201306756. ISSN 1433-7851.
- ^ Wickens, Zachary K.; Skakuj, Kacper; Morandi, Bill; Grubbs, Robert H. (2014-01-13). "Catalyst-Controlled Wacker-Type Oxidation: Facile Access to Functionalized Aldehydes". Journal of the American Chemical Society. 136 (3): 890–893. doi:10.1021/ja411749k. ISSN 0002-7863.
- ^ Kim, Kelly E.; Li, Jiaming; Grubbs, Robert H.; Stoltz, Brian M. (2016-09-30). "Catalytic Anti-Markovnikov Transformations of Hindered Terminal Alkenes Enabled by Aldehyde-Selective Wacker-Type Oxidation". Journal of the American Chemical Society. 138 (40): 13179–13182. doi:10.1021/jacs.6b08788. ISSN 0002-7863.
- ^ a b Hartwig, John F. (2010). Organotransition Metal Chemistry: From Bonding to Catalysis. USA: University Science Books. pp. 717–734. ISBN 978-1-81389-53-5.
{{cite book}}: Check|isbn=value: length (help) - ^ Baeckvall, Jan E.; Bystroem, Styrbjoern E.; Nordberg, Ruth E. (1984-11). "Stereo- and regioselective palladium-catalyzed 1,4-diacetoxylation of 1,3-dienes". The Journal of Organic Chemistry. 49 (24): 4619–4631. doi:10.1021/jo00198a010. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Hosokawa, Takahiro; Miyagi, Shyogo; Murahashi, Shunichi; Sonoda, Akio (1978-7). "Oxidative cyclization of 2-allylphenols by palladium(II) acetate. Changes in product distribution". The Journal of Organic Chemistry. 43 (14): 2752–2757. doi:10.1021/jo00408a004. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Baeckvall, Jan E.; Granberg, Kenneth L.; Andersson, Pher G.; Gatti, Roberto; Gogoll, Adolf (1993-9). "Stereocontrolled lactonization reactions via palladium-catalyzed 1,4-addition to conjugated dienes". The Journal of Organic Chemistry. 58 (20): 5445–5451. doi:10.1021/jo00072a029. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Timokhin, Vitaliy I.; Stahl, Shannon S. (2005-12). "Brønsted Base-Modulated Regioselectivity in the Aerobic Oxidative Amination of Styrene Catalyzed by Palladium". Journal of the American Chemical Society. 127 (50): 17888–17893. doi:10.1021/ja0562806. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Larock, Richard C.; Hightower, Timothy R.; Hasvold, Lisa A.; Peterson, Karl P. (1996-01). "Palladium(II)-Catalyzed Cyclization of Olefinic Tosylamides". The Journal of Organic Chemistry. 61 (11): 3584–3585. doi:10.1021/jo952088i. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help)
